Org. Synth. 1925, 5, 93
DOI: 10.15227/orgsyn.005.0093
SEMICARBAZIDE SULFATE
Submitted by A. W. Ingersoll, L. J. Bircher, and M. M. Brubaker.
Checked by Henry Gilman and L. C. Heckert.
1. Procedure
(A) Apparatus.—The reduction is carried out in a 15 by 23 cm. battery jar (Note 1), which is surrounded by a vessel suitable for a cooling bath. The bottom of the battery jar is covered with mercury, which serves as the cathode of the cell. The anode is a heavy lead coil separated from the catholyte by being suspended in a porous cup. This cup is set upon a support which holds it just above the cathode surface and does not appreciably diminish the latter (Note 2). The cathode is connected with the circuit by means of a glass tube partially filled with mercury and carrying a small piece of platinum wire sealed through the lower end. An efficient mechanical stirrer is provided in the catholyte. The current used in these experiments was 110 d.c., with a field rheostat, having a resistance of 8 ohms and a capacity of 20 amperes, for controlling the current. Any source of current supplying 30–110 volts and 15–20 amperes and any rheostat capable of carrying this current may be used. An ammeter reading to at least 15 amperes is connected in the circuit. Several reduction cells of the size described above may be run at one time, if they are connected in series. Figure 25 shows the general set-up.
Fig. 25.
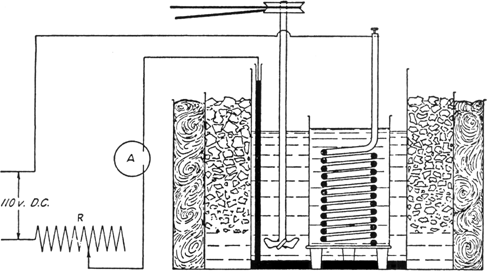
(B) Reduction of Nitrourea.—After the apparatus is assembled, 600 cc. of 20 per cent sulfuric acid (Note 3) is placed in the battery jar, and the lead anode in the porous cup is also covered with acid of the same strength. The cell is surrounded by an ice-salt bath, the stirrer is started, and, while the solution is cooling, 50 g. (0.48 mole) of nitrourea (Note 4) is added to the catholyte. A thermometer is placed in the catholyte, and when the temperature drops to +5° the current is turned on as follows: with the rheostat set for maximum resistance, the current is switched on, then the rheostat is gradually adjusted until the current flowing through the cell is about 0.06 ampere per square centimeter of cathode surface (Note 5). The cell must be efficiently cooled with ice and salt so as to keep the temperature at all times below 10° (Note 6). The reduction requires five to six hours. The nitrourea is quite insoluble but gradually dissolves as it is reduced. The foam and clumps of solid are worked down into the liquid occasionally. When almost all the nitrourea is in solution, some of the catholyte should be drawn up with a pipette and used to wash down the nitrourea that adheres to the walls of the vessel. The reduction is continued for about ten to twenty minutes after the nitrourea has dissolved. When the reduction is complete, as is indicated by a marked evolution of hydrogen, the current is shut off, and the porous cup is washed down with a little distilled water and removed.
The solution of
semicarbazide sulfate is removed from the cell and filtered
(Note 7). The filtered solution is concentrated under reduced pressure on a
water bath (Note 8) to a volume of 125–150 cc. Meanwhile, considerable
semicarbazide sulfate will have crystallized. The mixture is cooled thoroughly in ice and the crystals are collected on a
hardened filter paper, or better, on a
filtros plate (see
Note 5, on p. 11), and washed several times with
absolute alcohol to remove
sulfuric acid. The crystals are dried on clay plate or paper. The yield is
50–57 g. (
61–69 per cent of the theoretical amount) of a product melting at
144–145° with decomposition.
2. Notes
1.
This is a commercial size. The internal diameter of these battery jars is 15–17 cm. Any
sturdy glass vessel of the same dimensions may be used.
2.
The lead anode should have about the same surface area as the cathode. In these experiments the porous cup used was 8 by 21 cm., but slightly larger sizes would do. A
three-legged desiccator plate makes a convenient support for the cup. The latter should be immersed as deeply as possible in the catholyte, to decrease the resistance and consequent heating in the cell. It should not be more than 4–5 mm. thick, or the resistance will be too great and excessive heating will occur. Another convenient arrangement for the reduction cell is to divide the battery jar into two compartments by sealing in a diaphragm of thin cork-pine wood by means of paraffin. This offers very little resistance to the passage of the current and allows the reduction to be carried on very rapidly. A filtros plate may also be used as a diaphragm to separate the two solutions.
3.
For runs of this size,
sulfuric acid as dilute as 15 per cent may be used with good results. In larger runs or in runs using moist
nitrourea, the 20 per cent acid is preferable.
4.
Various grades of
nitrourea (p. 417) may be used in the reduction without noticeably affecting the purity of the
semicarbazide sulfate. In order to obtain the yields given in the procedure, the
nitrourea was well washed, sucked dry on the filter, and air-dried. The moist material obtained after filtration is equally good (see
(Note 3)). Runs in which
60–70 g. of nitrourea was used gave slightly lower yields than those in which 50 g. was used.
5.
The current varies somewhat with changes in temperature and concentration and must be adjusted occasionally. If very efficient cooling can be had, currents as high as 0.07 ampere per square centimeter may be used. The current efficiency and speed of reduction are decreased with currents below 0.06 ampere per square centimeter. A current of 13.5 amperes was satisfactory for the cell described.
6.
The yield falls off rapidly at temperatures above 10°. The control of temperature is much easier if the porous cup is immersed as deeply as possible in the catholyte
(Note 2).
7.
If another run is to be made immediately, the reduced liquor may be removed by means of a
siphon or pipette. If it is decanted, a little vaseline rubbed on the edge of the vessel will aid in pouring off the aqueous liquor from the
mercury.
8.
The evaporation must be conducted on a water bath whose temperature does not exceed 50–55°, in order to avoid decomposition of the product. The evaporation may also be conveniently carried out by placing the solution in a beaker, warming to 50–55°, and blowing air over the surface of the solution. This takes a somewhat longer time but requires no watching.
3. Discussion
Salts of semicarbazide can be prepared by the action of
potassium cyanate on
hydrazine sulfate;
1 by the action of
hydrazine hydrate on
urea;
2 by heating
hydrazine ammonium carbonate;
3 by the reduction of
nitrourea with
zinc dust and hydrochloric acid;
4 and by electrolytic reduction with iron cathodes and
ammonium chloride solution,
5 with tin cathodes and
sulfuric acid solution,
6 with lead cathodes and
hydrochloric acid solution,
7 and with cathodes of copper, nickel, lead, and mercury in
hydrochloric or sulfuric acid solution.
8
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Salts of semicarbazide
hydrochloric or sulfuric acid
alcohol (64-17-5)
sulfuric acid (7664-93-9)
hydrochloric acid (7647-01-0)
ammonium chloride (12125-02-9)
hydrogen (1333-74-0)
mercury (7439-97-6)
zinc (7440-66-6)
urea (57-13-6)
hydrazine hydrate (7803-57-8)
Hydrazine sulfate (10034-93-2)
Nitrourea (556-89-8)
potassium cyanate (590-28-3)
Semicarbazide sulfate
hydrazine ammonium carbonate
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved