Org. Synth. 1954, 34, 79
DOI: 10.15227/orgsyn.034.0079
1-PHENYLPIPERIDINE
[Piperidine, 1-phenyl]
Submitted by A. N. Bourns, H. W. Embleton, and Mary K. Hansuld
1.
Checked by R. T. Arnold, William E. Parham, and Carl Serres.
1. Procedure
The apparatus (
Fig. 15) consists of a
reaction tube, 100 cm. long and 3 cm. in diameter, provided with an inlet tube to which a graduated dropping funnel is connected. The lower end of the reaction tube is fitted with a
Friedrichs condenser and
receiver. A
thermocouple well, concentric with the reaction tube, is passed through the upper end by means of a standard ground-glass joint. The tube is packed with
200 ml. of 4- to 8-mesh alumina (Note 1) held in place by a plug of glass wool supported by indentations in the tube, followed by 100 ml. of marble chips which serve as a vaporizer and preheater for the reactants. The reaction tube is supported in an
electrically heated furnace (Note 2) extending from the inlet tube to the joint connecting the condenser.
Fig. 15.
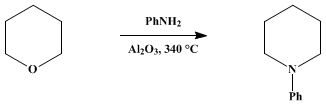
The reaction tube is heated to 330–340°
(Note 3) and is swept out with a stream of
nitrogen gas. A solution of
258 g. (3 mole) of tetrahydropyran (Note 4) and
559 g. (6 mole) of aniline is then placed in the graduated funnel and introduced into the reaction tube at a rate of 90–100 ml. per hour. The product, which is collected in a
1-l. flask containing
10 g. of sodium chloride, consists of a light-yellow oil and a lower aqueous layer. After the addition of the reactants is complete, a slow stream of
nitrogen is passed through the reaction tube for 20–30 minutes to remove any product adsorbed on the catalyst. The lower aqueous layer is separated and discarded, and the upper organic layer is dried over
sodium hydroxide pellets. The product is fractionated under reduced pressure using a
Whitmore-Lux column2 (Note 5) only to remove the small amount of unchanged
tetrahydropyran and excess
aniline. The column is permitted to drain, and the residue is distilled from an
ordinary Claisen flask under reduced pressure.
1-Phenylpiperidine is obtained as a colorless or very light-yellow liquid, b.p.
123–126°/12.5 mm.,
133–136°/21 mm.,
nD25 1.5603. The yield is
403–435 g. (
83–90% based on
tetrahydropyran)
(Note 6).
2. Notes
1.
The catalyst employed was Alcoa Activated
Alumina (Grade F-1, 4- to 8-mesh) from the Aluminum Company of America. A fresh catalyst is brought to a condition of maximum activity by passing a slow stream of air through the catalyst bed for 94 hours at 390–405°. Without this pretreatment, yields are
5–10% lower than those reported here. The catalyst is reactivated after each run by passing air through it for 39 hours at 390–405°.
2.
The furnace may be of the construction described in a previous volume.
3 It is desirable, although not essential, to provide separately controlled heating elements for each of the two packed zones of the reaction tube.
3.
The temperature of the furnace is measured by a thermocouple which can be moved to various positions in the thermocouple well. The catalyst temperature should be maintained at 325–345°, although it may be as low as 320° at the ends of the catalyst zone, depending unon the construction of the furnace.
4.
Eastman Kodak Company practical grade tetrahydropyran may be used without purification.
5.
A column of equivalent efficiency may be employed.
6.
1-m-Tolylpiperidine (b.p.
141–143°/16 mm.,
nD25 1.5535) and
1-p-tolylpiperidine (b.p.
140–143°/15 mm.,
nD25 1.5509) may be prepared in 85–90% yield by a similar procedure.
1-o-Tolylpiperidine (b.p.
123–125°/15 mm.,
nD25 1.5391) is obtained in
60% yield under similar reaction conditions, but it is necessary to fractionate the product in order to obtain pure material.
3. Discussion
1-Phenylpiperidine has been prepared by warming
aniline with
1,5-dibromopentane;
4,5 heating
5-anilino-1-bromopentane;
6 the dehydration of
5-anilino-1-pentanol over
alumina;
7 the electrolytic reduction of
N-phenylglutarimide;
8 the catalytic hydrogenation of
1-phenyl-3-hydroxypyridinium chloride;
9 the action of
bromobenzene on
piperidine in the presence of
lithium10 or sodium amide;
11 the reaction of
fluorobenzene,
1-methylpiperidine, and
phenyllithium;
12 the action of
diphenylsulfone on
piperidine in the presence of
sodamide;
13 the diazotization and deamination of
1-(2-aminophenyl)piperidine14 and of
1-(4-aminophenyl)piperidine;
15 the reduction of
N-phenylglutarimide with
lithium aluminum hydride;
16 the reaction of
pentamethylene glycol dibenzenesulfonate with
aniline;
17 the action of
lithium piperidide on
chlorobenzene;
18 and the present method.
19
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
alumina
lithium piperidide
aniline (62-53-3)
sodium hydroxide (1310-73-2)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
chlorobenzene (108-90-7)
diphenylsulfone (127-63-9)
piperidine (110-89-4)
1,5-dibromopentane (111-24-0)
bromobenzene (108-86-1)
Tetrahydropyran (142-68-7)
Phenyllithium (591-51-5)
Fluorobenzene (462-06-6)
sodamide (7782-92-5)
lithium aluminum hydride (16853-85-3)
1-Phenylpiperidine,
Piperidine, 1-phenyl (4096-20-2)
5-anilino-1-bromopentane
5-anilino-1-pentanol
N-phenylglutarimide
1-phenyl-3-hydroxypyridinium chloride
1-methylpiperidine (626-67-5)
1-(2-aminophenyl)piperidine (39643-31-7)
1-(4-aminophenyl)piperidine (2359-60-6)
pentamethylene glycol dibenzenesulfonate
1-m-Tolylpiperidine
1-p-tolylpiperidine
1-o-Tolylpiperidine (7250-70-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved