Org. Synth. 2002, 78, 254
DOI: 10.15227/orgsyn.078.0254
1-OXO-2-CYCLOHEXENYL-2-CARBONITRILE
[
1-Cyclohexene-1-acetonitrile, 6-oxo-
]
Submitted by Fraser F. Fleming
1
and Brian C. Shook
1
.
Checked by Anne F. Vergne and Marvin J. Miller.
1. Procedure
Caution! Ozone is extremely toxic and can react explosively with certain oxidizable substances. Ozone also reacts with some compounds to form explosive and shock-sensitive products. Ozone should only be handled by individuals trained in its proper and safe use and all operations should be carried out in a well-ventilated fume hood behind a protective safety shield. [Note added September 2009].
1-Oxo-2-cyclohexenyl-2-carbonitrile
.
A dry, 100-mL, round-bottomed flask containing a magnetic
stirring bar is fitted with an inlet adapter for
ozonolysis (Note 1, Figure 1) and charged
with
1-cyclopenteneacetonitrile (5.0
g, 46.7 mmol, Note 2)
and
60 mL of dry dichloromethane
(Note 3). A gentle stream of dry ozone
is passed through the solution and the flask is immediately cooled to −78°C
(Note 4). Ozonolysis is continued until the distinctive blue
color of excess
ozone
is
first observed, ozonolysis is then terminated, and the excess ozone is removed by
purging with a stream of
nitrogen
for 5-10 min. The solution is allowed to warm to room temperature, the ozonolysis
adapter
is replaced with a rubber septum, and neat
dimethyl sulfide (3.9 g, 62.1 mmol,
Note 5) is added via syringe. The
solution is allowed to stir at room temperature for 36 hr during which time the solution
changes in color from a pale yellow to dark red. The resulting solution is concentrated
under reduced pressure using a rotary evaporator, and the resulting
thick, red syrup is diluted with
40 mL of
ethyl acetate
and washed with water (3 × 25 mL, Note
6). The aqueous phase is extracted with
ethyl acetate (3 × 25 mL),
the organic phases are combined, rinsed with brine
in order to remove all DMSO, dried (MgSO4), filtered, and concentrated.
The residual red oil (5.55 g,
98%) contains only trace impurities
and can be used without purification in most cases (Note 7).
If required, further purification is achieved by rapid radial chromatography (Note 8) on a 2-mm plate using the solvent delivery tip designed for a
4-mm plate and eluting with
50%
ethyl
acetate-hexane
(Note 9).
The desired fractions are combined and concentrated to provide
1-oxo-2-cyclohexenyl-2-carbonitrile
(4.8 g, 85% yield) as a pink oil (Note 10).
Figure 1
2. Notes
1.
A
short length of glass tubing (I. D. = 3 mm)
is submerged (1 cm) beneath the surface of the solvent and the outlet tubing is immersed
in a saturated solution of
potassium iodide
.
2
2.
1-Cyclopenteneacetonitrile
was purchased from Oakwood Products
and purified by
Kugelrhor distillation (50-60°C at 5 mm) prior to ozonolysis.
1-Cyclopenteneacetonitrile
from other suppliers (Aldrich and Acros)
was treated similarly.
3.
Dichloromethane
was distilled from
calcium hydride
.
4.
The
ozone
is dried by passing the gas through a trap containing
concentrated
sulfuric acid
. The ozonolysis is initiated prior to
cooling to −78°C to prevent a vacuum from forming that would otherwise cause
the
potassium iodide
solution
to be drawn into the reaction flask.
5.
The
dimethyl sulfide
was purchased from Aldrich Chemical Company, Inc.
,
and used without purification.
6.
This extraction procedure ensures removal of the
dimethyl
sulfoxide
that is produced in the reaction.
7.
The crude material reacts conjugately with
phenylmagnesium
bromide
affording the addition product in 49% yield compared
to 55% obtained using chromatographically pure
1-oxo-2-cyclohexenyl-2-carbonitrile
.
3
8.
Rapid radial chromatography is essential since column chromatography
results in significant irreversible adsorption of
1-oxo-2-cyclohexenyl-2-carbonitrile
.
For example, column chromatography of a relatively pure
3.0-g sample afforded only
0.5
g of pure
1-oxo-2-cyclohexenyl-2-carbonitrile
.
However, the checkers observed that a sample, purified as described by radial chromatography,
survived flushing through a plug of silica gel.
9.
The crude oxonitrile is dissolved in
10
mL of 50%
ethyl acetate-hexane
solution to afford a homogeneous solution. Incomplete removal of DMSO
(Note 6) results in a two-phase mixture that, if loaded directly onto
the silica plate, results in a diminished yield through partial absorption of the
oxonitrile on the silica gel.
10.
The product solidifies on standing at −4°C and can be stored
neat at this temperature for several weeks or as a solution in
benzene
for at least two months. The spectral data are as follows: IR (film) cm
−1: 2233, 1698,
1615
;
1H
NMR δ: 2.10 (br quintet, 2 H, J = 6), 2.53-2.61 (m, 4 H),
7.75 (t, 1 H, J = 4.2)
;
13C
NMR δ: 21.2 (t), 26.3 (t), 36.9 (t),
114.0 (s), 117.3 (s), 163.4 (d), 192.0
(s)
. MS m/e 122
(M + H
+).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Highly electron-deficient alkenes are valuable reactants
for both cycloaddition and conjugate addition reactions. The demand for highly reactive
cycloalkenones has resulted in several syntheses of cycloalkenones containing an additional
electron-withdrawing group on the α-carbon.
4 Oxonitriles represent an ideal
compromise between high reactivity and stability toward storage and chromatography.
5
The conversion of
cyclopentenacetonitrile
to
1-oxo-2-cyclohexenyl-2-carbonitrile
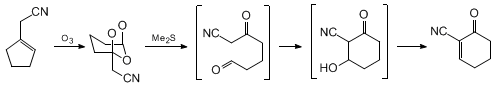
proceeds by a domino ozonolysis-aldol sequence (Scheme 1).
6
Isolation and characterization of the ozonide
7
preclude the direct cyclization of the intermediate carbonyl oxide and establish that
the cyclization occurs after the addition of
dimethyl
sulfide
. Subsequent formation of the
bis-oxonitrile
(
1H NMR analysis) occurs rapidly and is followed by a slower cyclization-dehydration
to afford
1-oxo-2-cyclohexenyl-2-carbonitrile
.
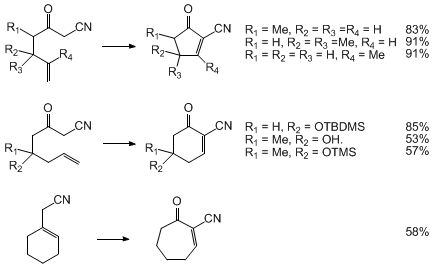
The domino ozonolysis-aldol sequence represents a general method for preparing
5-, 6-, and 7-membered cyclic oxonitriles (Scheme 2).
6
Cyclization to 6-membered ring oxonitriles proceeds as for the parent system,
1-oxo-2-cyclohexenyl-2-carbonitrile
,
whereas the corresponding 5- and 7-membered oxonitriles are less prone to cyclization.
Cyclization of the 5- and 7-membered oxonitriles requires exposure of the aldehyde
intermediates to acid or base in order to promote the geometrically more challenging
cyclization.
6
1-Oxo-2-cyclohexenyl-2-carbonitrile
is an exceptional Michael acceptor that reacts conjugately with Grignard reagents
without catalysis.
3 In most cases the intermediate
enolates are silylated to afford substituted β-siloxy unsaturated nitriles, several
of which are excellent precursors to cis- and trans-decalins (Scheme 3).
8 For example, unmasking
the latent ketone enolate of siloxy unsaturated nitriles provides the cis-decalin
in excellent yield while the trans-decalin is obtained from the same precursor by
cyclizing the corresponding nitrile anion.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
1-Oxo-2-cyclohexenyl-2-carbonitrile:
1-Cyclohexene-1-carbonitrile,
6-oxo- (11); (91624-93-0)
1-Cyclopenteneacetonitrile:
1-Cyclopentene-1-acetonitrile
(9); (22734-04-9)
Ozone (8,9); (10028-15-6)
Dimethyl sulfide:
Methyl sulfide
(8);
Methane, thiobis- (9); (75-18-3)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved