Intramolecular Coupling for General Synthesis of Backbone Substituted Bicyclo[1.1.1]pentyl Boronates
1. Procedure (Note 1)
A. 3,3-Dimethoxy-1-phenylcyclobutane-1-carbonitrile (1). A 1000-mL single-necked round-bottomed flask is equipped with a 6.4 cm Teflon-coated magnetic stir bar. The apparatus is flame-dried under vacuum, cooled to 25 ℃ and refilled with argon three times (Figure 1A). The flask is charged with sodium hydride (60%, 500 mmol, 20.0 g, 2.5 equiv) (Note 2). Then N,N-dimethylformamide (470 mL) (Note 3) is added into the flask, and the mixture is cooled to 0 ℃ in an ice-water bath and stirred for 15 min at 280 rpm (Figure 1B). Then an N,N-dimethylformamide (30 mL) solution of benzyl cyanide (200 mmol, 23.4 g, 23.1 mL, 1.0 equiv) (Note 4) is added dropwise into the flask through a dropping funnel at 0 ℃ over a period of 40 min (Note 5) (Figure 1C). After benzyl cyanide is added, the reaction system is allowed to warm up to room temperature (22-26 ℃) and stirred at 280 rpm until the hydrogen release stops (typically 0.5 h) (Figure 1D). Then 1,3-dibromo-2,2-dimethoxy-propane (240 mmol, 62.8 g, 1.2 equiv) (Note 6) is added through a feeding funnel into the flask at room temperature (22-26 ℃), and the mixture turns into a dark green suspension (Figure 1E). The reaction is allowed to stir at the ambient temperature at 280 rpm for another 16 h (Note 7). Reaction progress is monitored by TLC analysis (Note 8) (Figure 1F).
After benzyl cyanide is totally consumed as determined by TLC analysis, the reaction is cooled to 0 ℃ in an ice-water bath. Excess sodium hydride is quenched by dropwise addition of water (50 mL x 2) (Note 9) through a 60 mL syringe over 30 min (Figure 1G). The stir bar is removed, and the reaction mixture is concentrated to remove DMF solvent by rotary evaporation (30 ℃, 45 mmHg to 50 ℃, 15 mmHg) (Figure 1H). Next, the remaining reaction mixture (about ~150 mL) is diluted with water (400 mL) and diethyl ether (250 mL) (Note 10), and the solution is transferred to a 1000-mL separatory funnel (Figure 1I). The aqueous phase is separated (Note 11) and extracted with diethyl ether (3 x 150 mL). The combined organic layers are washed with water (100 mL) and saturated NaCl solution (100 mL) and then dried over 60 g of anhydrous Na2SO4. The solution is filtered through Celite (60 g, prewet with 100 mL diethyl ether) (Note 12) in a 350-mL large porosity sintered glass funnel into a 1000 mL round-bottomed flask using diethyl ether as eluent (300 mL) (Figure 1J). To the reaction mixture is added 178 g silica gel (Note 13) and concentrated by rotary evaporation (30℃, 375 mmHg to 30 ℃, 15 mmHg) to afford a homogeneous yellow solid (Figure 1K), which is loaded onto a chromatography column (7.0-cm width and 50-cm height), prepared from 430 g silica gel (Note 14) and with 500 mL of hexanes (Note 15) (Figure 1L). At this point, fraction collection (250-mL fractions) begins, and elution is continued with 500 mL hexanes, 500 mL of 10% EtOAc-hexane (9: 1 hexanes:EtOAc) (Note 16) and then 3.5 L of 20% EtOAc-hexane (4: 1 hexanes:EtOAc). The desired product is obtained in fractions 12-16 (Figure 1M) (Note 17), which are concentrated by rotary evaporation (30 ℃, 175 mmHg to 30 ℃, 15 mmHg) to afford a yellow solid as the crude product (Figure 1N).
The yellow solid is redissolved in hot EtOAc (7.0 mL) (Figure 1O) and then allowed to cool to room temperature (23-26 ℃) until the solids precipitate. Additional hexanes (40.0 mL) are added dropwise at the rate of 40 mL/h. This solution is cooled at 0 ℃ in an ice-water bath and stirred for 1 h, and the resulting solids (Figure 1P) are collected by suction filtration on a Büchner funnel. The residual solid is further washed with 14% EtOAc-hexane (40 mL) (6:1 hexanes:EtOAc), and then transferred to a 100-mL round-bottomed flask and dried at 1 mmHg for 2 h to provide white solid as the product (19.1 g, 44%) (Notes 18, 19, and 20) (Figure 1Q).
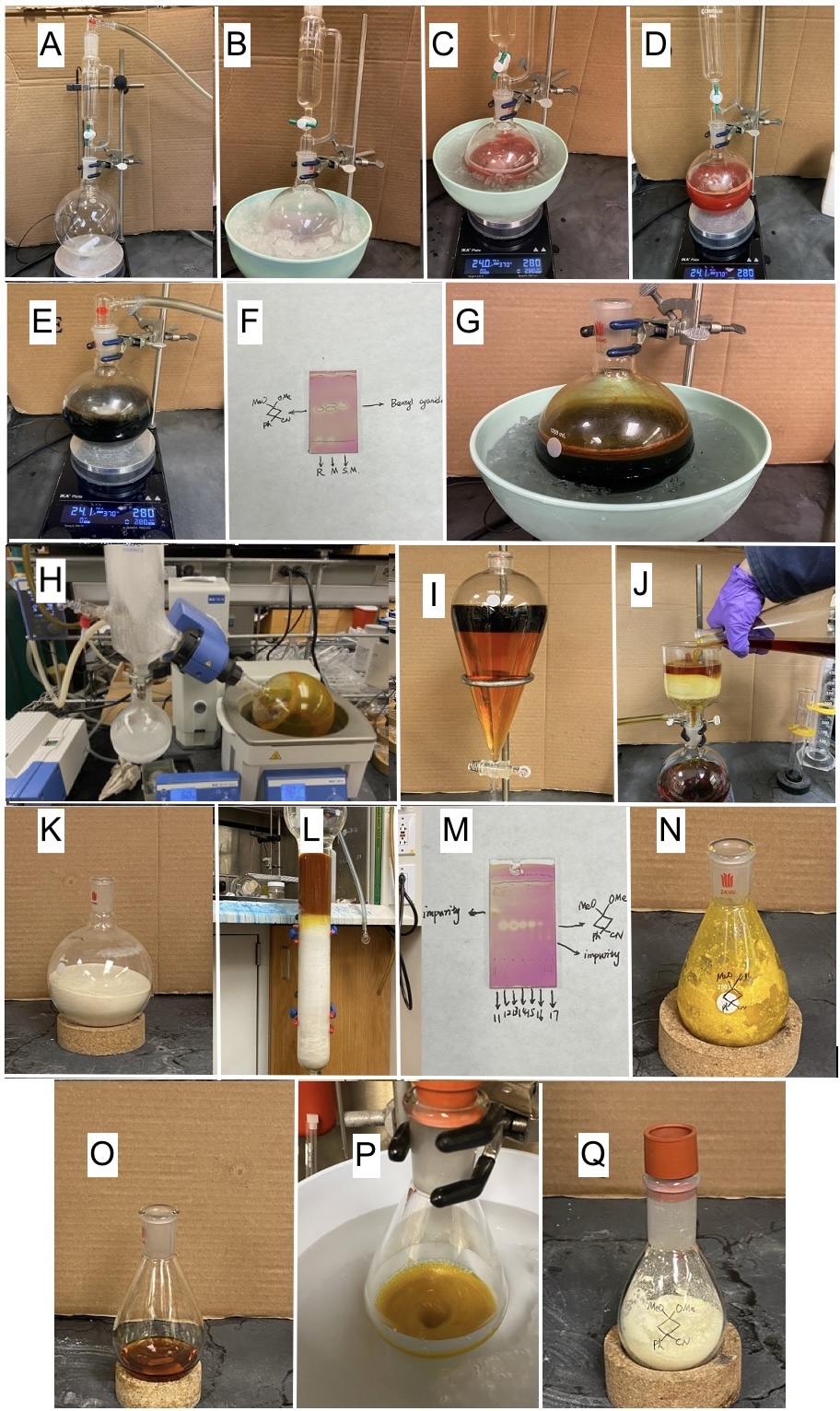
Figure 1. Synthesis of Compound 1: (A) Reaction equipment setup; (B) Reaction set-up containing NaH solution in DMF; (C) Benzyl cyanide is added; (D) The reaction is warmed up to room temperature; (E) 1,3-Dibromo-2,2-dimethoxypropane is added; (F) TLC plate of the reaction mixture; (G) Reaction mixture is quenched; (H) Reaction mixture is concentrated; (I) Aqueous and organic layers; (J) Dried organic layers are filtered; (photos provided by authors) Figure 1 (continued). K) Reaction crude with silica gel for chromatographic purification; (L) Column chromatography with reaction crude; (M) TLC of fractions; (N) Concentrated crude oil after chromatography purification; (O) Oil is dissolved in hot EtOAc-hexane solvent. (P) Solids in the reaction crude after being cooled; (Q) Pure product 1. (photos provided by authors)
C. N'-(3-(Cyclopentyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methyl)-3-phenylcyclobutylidene)-2,4,6-trimethylbenzenesulfonohydrazide (3). An oven-dried 250-mL single-necked (24/40 joint) round-bottomed flask is equipped with a 3 cm Teflon-coated football-shape magnetic stir bar. The apparatus is charged with 2 (12 mmol, 5.0 g, 1.0 equiv), cyclopentyl boronic acid (4.1 g, 36 mmol, 3.0 equiv) (Note 32), and cesium carbonate (11.7 g, 36 mmol, 3.0 equiv) (Note 33) under an atmosphere of argon (Figure 3A). Chlorobenzene (60 mL) (Note 34) is added to the reaction, and the flask is heated in a 100 ℃ oil-bath and stirred at 650 rpm (Figure 3B). After heating for 2 h, the nitrogen is totally released (Note 35), and it is confirmed by TLC analysis that hydrazone is totally consumed (Figure 3C) (Note 36). The reaction mixture is cooled to room temperature (22-26 ℃) and pinacol (7.1 g, 60 mmol, 5.0 equiv) is added to the mixture under the argon atmosphere (Note 37). Then the reaction mixture is reheated in a 100 ℃ oil-bath and stirred at 650 rpm for another 30 min (Figure 3D). The boronic ester formation process is monitored by TLC analysis (Figure 3E) (Note 38). After the flask is re-cooled to room temperature (22-26 ℃), the reaction mixture is filtered through Celite (30 g, prewet with 50 mL diethyl ether), placed in a 150-mL large porosity sintered glass funnel, into a 500 mL round-bottomed flask using diethyl ether as eluent (200 mL) (Figure 3F). The filtered solution is concentrated by rotary evaporation (30 ℃, 225 mmHg to 35 ℃, 7.5 mmHg) to remove excess diethyl ether and chlorobenzene (Figure 3G).
The concentrated crude reaction is dissolved in 50 mL of acetonitrile (Note 39), to which is added HCl solution (48 mmol, 3 M, 16 mL, 4.0 equiv) (Note 40). The solution is stirred at 400 rpm for 60 min (Figure 3H). The hydrolysis is monitored by TLC analysis (Note 41) (Figure 3I). After the acetal is totally consumed, as determined by TLC analysis, the crude reaction is concentrated by rotary evaporation (30 ℃, 225 mmHg to 32 ℃, 7.5 mmHg) to remove excess acetonitrile (Figure 3J). Diethyl ether (100 mL) and saturated brine (50 mL) are added to the reaction mixture, and this solution is transferred to a 500-mL separatory funnel (Figure 3K). The aqueous layer is separated and further extracted with diethyl ether (3 x 50 mL). The combined organic layers are dried over anhydrous Na2SO4 (50 g), filtered through Celite (20 g, prewet with 50 mL diethyl ether) that is placed a 150-mL large porosity sintered glass funnel into a 500 mL round-bottomed flask. Diethyl ether (50 mL) is used to wash the Celite, and the resulting solution is concentrated by rotary evaporation (30 ℃, 225 mmHg to 32 ℃, 7.5 mmHg) to remove excess solvent.
After solvent removal, a 3 cm Teflon-coated football-shape magnetic stir bar, 2-mesitylenesulfonyl hydrazide (18 mmol, 3.9 g, 1.5 equiv) and toluene (50 mL) (Note 42) are added into the flask, and the reaction mixture is stirred at room temperature (23-26 ℃) at 500 rpm for 12 h (Figure 3L). The hydrazone formation process is monitored by TLC analysis (Note 43) (Figure 3M). After the ketone is confirmed to be totally consumed, the reaction crude is concentrated by rotary evaporation (30 ℃, 225 mmHg to 35 ℃, 7.5 mmHg) to remove excess toluene.
The crude product is dissolved in methylene chloride (100 mL) and then silica gel (23.7 g) is added. The mixture is concentrated in vacuo to afford a homogeneous yellow solid. The solid is loaded onto a chromatography column (4.6 x 45.7 cm) that contains 104.5 g silica gel, which had been wet-packed with 500 mL hexanes (Figure 3O). The column is eluted within 30 min (Note 44) with 550 mL of hexanes: ethyl acetate: methylene chloride (20:1:1). At that point, elution is continued sequentially with 525 mL of hexanes: ethyl acetate: methylene chloride (18:2:1), and then 550 mL of hexanes: ethyl acetate: methylene chloride (18:3:1). The desired product is obtained in fractions 41-76 (Note 45) (Figure 3P), which are concentrated by rotary evaporation (30 ℃, 275 mmHg to 30 ℃, 15 mmHg) to afford a yellow solid collected in a 100-mL single-necked flask. Hexanes (40 mL) are added into the flask, and the mixture is stirred at room temperature (23-26 ℃) for 0.5 h (Figure 3Q). The resulting solids (Figure 3R) are collected by suction filtration on a Büchner funnel. The residual solid is further washed with hexanes (50 mL) , and then transferred to a 20 mL vial and dried at 1 mmHg for 2 h to provide the white solid as the product (2.62 g, 40%) (Notes 46, 47, and 48) (Figure 3S).

Figure 3. Synthesis of Compound 3: (A) Reaction setup with solid reagents; (B) Reaction mixture being heated with nitrogen released; (C) TLC plate of the boronic acid; (D) Reaction mixture forming boronic pinacol ester; (E) TLC plate of the boronic pinacol ester; (F) Filtered reaction crude; (G) Excess solvent is removed; (H) Hydrolysis reaction; (I) TLC plate of the crude hydrolysis; (J) Excess acetonitrile is removed; (K) Aqueous and organic layers; (L) Reaction mixture forming sulfonyl hydrazone; (M) TLC plate of sulfonyl hydrazone formation; (N) Crude reaction product on silica gel; (O) Column chromatography with reaction crude; (P) TLC plates of fractions; (Q) purified product washed with hexanes; (R) Filtration of washed products; (S) Pure product 3. (photos provided by authors)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
sodium hydride,
benzyl cyanide,
1,3-dibromo-2,2-dimethoxy-propane,
N,N-dimethylformamide,
diethyl ether,
NaCl,
Na2SO4, silica gel,
Rochelle salt, hexanes,
ethyl acetate,
methylene chloride,
DIBAL-H,
Celite,
2-mesitylenesulfonyl hydrazide,
cyclopentyl boronic acid,
cesium carbonate,
chlorobenzene,
pinacol,
acetonitrile,
HCl solution, brine,
toluene,
dioxane, and
1,3,5-trimethoxybenzene.
2.
Sodium hydride (60%, dispersion in Paraffin Liquid) was purchased from TCI and used as received immediately upon receipt.
3.
N,N-Dimethylformamide (99.8%, Super Dry, with molecular sieves) was purchased from Acros Organics and used as received. The checkers observed that the use of fresh and dry
DMF was important for the success of the reaction.
4.
Benzyl cyanide (98%) was purchased from Sigma-Aldrich and used as received.
5. Hydrogen was released during the addition of
benzyl cyanide. Therefore, the speed of addition of
benzyl cyanide should be slow. With
benzyl cyanide added, the color of reaction mixture turned from grey to red suspension through pale yellow for a short time (Figure 1C).
6.
1,3-Dibromo-2,2-dimethoxypropane (98%) was purchased from BLD Pharmatech Co. and used as received.
7. Hydrogen gas evolved as the reaction warmed to room temperature (22-26 ℃) and continued with slight exotherms (Figure 1D).
8. An aliquot (0.1 mL) was removed and mixed with sat. NH
4Cl (1 mL) and
diethyl ether (1 mL), and the contents were thoroughly mixed. The organic layer was used for TLC monitoring, and reaction progress was determined by TLC analysis on silica gel using 1:4
EtOAc: hexanes as eluent and visualization with KMnO
4 (
Rf = 0.55). The reaction was determined to be completed when
benzyl cyanide was totally consumed (Figure 1F).
9. Additional hydrogen bubbles were also released during quenching the reaction with water. Thus, the speed of adding water to quench reaction should be controlled.
10.
Diethyl ether (99.0%) was purchased from Fisher and used as received.
11. The interface between the dark aqueous and the organic phase can be difficult to discern.
12.
Celite (545 Filter Aid, not acid-washed) was purchased from Fisher and used as received.
13. SiliaFlash P60 (particle size 0.040-0.063 mm) was purchased from SiliCycle and used as received.
14. The column is wet-packed using 430 g of silica and 1.0 L of hexanes.
15. Hexanes (98.5%) was purchased from Fisher and used as received.
16.
Ethyl acetate (99.5%) was purchased from Fisher and used as received.
17. Fractions (250 mL) containing the product were identified by TLC analysis (4:1 hexanes:
EtOAc as eluent,
Rf = 0.55). Fractions 12-14 contained only the desired product while fractions 15-16 contained the desired product and two impurities that can be visualized by UV irradiation and by KMnO
4 stain as two spots (Figure 1M). This impurity does not impact the trituration of the desired product
1, so these fractions were also collected. Test tubes containing the desired product were each rinsed with
EtOAc (2 x 2 mL), and the rinses were transferred into the collection flask.
18.
3,3-Dimethoxy-1-phenylcyclobutane-1-carbonitrile (
1): mp 53-55 ℃;
1H NMR
pdf (600 MHz, CDCl
3) δ: 7.51 - 7.45 (m, 2H), 7.43 - 7.37 (m, 2H), 7.35 - 7.29 (m, 1H), 3.28 (s, 3H), 3.18 (s, 3H), 3.13 - 3.07 (m, 2H), 2.76 - 2.70 (m, 2H) ppm;
13C NMR
pdf (151 MHz, CDCl
3) δ: 139.5, 129.1, 128.1, 125.9, 123.7, 98.1, 49.0, 48.7, 45.8, 31.2 ppm; HRMS (ESI+) calc. for C
13H
16NO
2 [M+H]
+ 218.1176, found 218.1177.
19. The purity of
1 was determined to be 97.9 wt% by qNMR
pdf using
1,3,5-trimethoxybenzene (Sigma-Aldrich, 99%) as the external standard.
20. A second run performed at full scale provided 20.9 g (48%) of
1 at 98.8 % purity.
21.
Methylene chloride(>99.9%, Optima
TM for HPLC and GC) was purchased from Fisher and dried through passing an activated alumina column.
22.
DIBAL-H (1.0 M in hexanes) was purchased from Sigma-Aldrich and used as received without further titration.
23. Control of the addition rate of
DIBAL-H is critical to the yield of compound
2. The yields are decreased when
DIBAL-H solution is added too quickly because of difficulties in controlling the reaction temperature. During the DIBAL addition, the internal temperature did not exceed 5 ℃ (Figures 2M and 2N).
24. An aliquot (0.1 mL) used for TLC monitoring was removed and diluted with 0.9 mL
methylene chloride, and reaction progress was determined by TLC analysis on silica gel using 1:4
EtOAc: hexanes as eluent and visualization with CAM stain (Figure 2D,
Rf = 0.36).
25.
Rochelle salt (
potassium sodium tartrate tetrahydrate) was purchased from BLD Pharmatech Co. The use of 1 mL of the saturated solution per 1.0 mmol of
DIBAL-H was found to be optimal. During the quench process, the internal temperature did not exceed 10 ℃ (Figure 2O)
26. 2-Mesitylsulfonyl hydrazide (98%) was purchased from Combi-Blocks and used as received.
27. An aliquot (0.1 mL) used for TLC monitoring was removed and diluted with 0.9 mL
methylene chloride, and reaction progress was determined by TLC analysis on silica gel with 1:4
EtOAc: hexanes as eluent and visualization with CAM stain (Figure 2H,
Rf = 0.43).
28. Fractions (50 mL) containing the product were identified by TLC analysis (4:1 hexanes:
EtOAc as eluent). Fractions 34-52 contained only the desired product, while fractions 28-33 contained the desired product and one impurity that can be visualized by UV irradiation and by CAM stain as two spots (Figure 2K). Fractions 53-68 contained the desired product and excess 2-mesitylene hydrazide. For fraction 33, 53, and 54, impurities do not impact the purity of the desired compound as judged by qNMR so these fractions were also collected. Test tubes containing the desired product were each rinsed with
EtOAc (2 x 3 mL), and the rinses were transferred into the collection flask.
29.
N'-((3,3-Dimethoxy-1-phenylcyclobutyl)methylene)-2,4,6-trimethylben-zenesulfonohydrazide (
2): mp 104-106 ℃;
1H NMR
pdf (600 MHz, Acetone-d
6) δ: 9.71 (s, 1H), 7.38 (s, 1H), 7.28 - 7.22 (m, 2H), 7.18 (ddt,
J = 8.1, 6.7, 1.3 Hz, 1H), 7.07 - 7.02 (m, 4H), 3.02 (s, 3H), 2.90 (s, 3H), 2.82 - 2.76 (m, 2H), 2.62 (s, 6H), 2.46 - 2.40 (m, 2H), 2.31 (s, 3H);
13C NMR
pdf (151 MHz, Acetone-d
6) δ: 153.8, 145.4, 143.3, 140.7, 134.4, 132.5, 129.2, 127.2, 127.1, 99.3, 48.3, 48.2, 41.9, 41.03, 23.4, 20.9; HRMS (ESI+) calc. for C
22H
28N
2O
4S [M+Na]
+ 439.1662, found 439.1666.
30. The purity of
2 was determined to be 99.0 wt% by qNMR
pdf using
1,3,5-trimethoxybenzene (Sigma-Aldrich, 99%) as the external standard.
31. A second run performed at full scale provided 16.1 g (50%) of
2 at 98.6% purity.
32.
Cyclopentyl boronic acid (97%) was purchased from Combi-Blocks and used as received.
33.
Cesium carbonate was purchased from BLD Pharmatech Co. and used as received.
34.
Chlorobenzene (99.9%) was purchased from Sigma-Aldrich and used as received.
35. As the reaction continues, nitrogen was released from the diazo intermediate. White solid, which could not dissolve in
chlorobenzene, was also observed in the reaction mixture (Figure 3B).
36. An aliquot (0.1 mL) used for TLC monitoring was removed and diluted with 0.9 mL
diethyl ether, and reaction progress was determined by TLC analysis on silica gel with 1:3
EtOAc: hexanes as eluent and visualization with CAM stain (Figure 3C,
Rf = 0.31). The side product, which was strongly visualized by UV light, was
(3,3-dimethoxycyclopent-1-en-1-yl)benzene (
5) (Figure 5), which was formed owing to self-rearrangement of diazo intermediate.
37.
Pinacol was purchased from TCI and used as received. The boronic acid formed in the first coupling step is sensitive to air at 100 ℃, thus operations before forming the boronic ester should be performed under argon atmosphere.
38. An aliquot (0.1 mL) used for TLC monitoring was removed and diluted with 0.9 mL
diethyl ether, and reaction progress was determined by TLC analysis on silica gel with 1:4
EtOAc: hexanes as eluent and visualization with CAM stain (Figure 3E,
Rf = 0.75).
39.
Acetonitrile (HPLC grade, 99.9%) was purchased from Fisher and used as received.
40. 3 M
HCl solution was prepared by slowly adding
HCl (36.5%-38% w.t., 125 mL) to 375 mL water. Be careful with the exotherms owing to
HCl dissolution.
HCl (36.5%-38% w.t.) was purchased from Fisher and used as received.
41. An aliquot (0.1 mL) used for TLC monitoring was removed and diluted with 0.9 mL
diethyl ether, and reaction progress was determined by TLC analysis on silica gel with 1:8 acetone: hexanes as eluent and visualization with CAM stain (Figure 3I,
Rf = 0.44). Cerium ammonium molybdate stain (CAM stain) was prepared by dissolving 12.5 g ammonium molybdate tetrahydrate and 5 g cerium ammonium sulfate dihydrate in 500 mL 10% H
2SO
4. The authors report that the spot which appears above the desired product is the impurity
5, and the spot which appears below the desired product is the hydrolyzed impurity
6,
3-phenylcyclopent-2-en-1-one (Figure 5).
Figure 5. Impurities 5 & 6 observed during the synthesis of 3
42.
Toluene (>99.8%) was purchased from Fisher and dried through passing an activated alumina column.
43. An aliquot (0.1 mL) used for TLC monitoring was removed and diluted with 0.9 mL
methylene chloride, and reaction progress was determined by TLC analysis on silica gel with 1:3
EtOAc: hexanes as eluent and visualization with CAM stain (Figure 3M,
Rf = 0.41).
44. The column was eluted with the solvents described in the procedure immediately after loading the sample on the column. The chromatographic separation took around 30 minutes. Eluting the column for longer time would result in a lower yield owing to sulfonyl hydrazone product crashing out and decomposition.
45. Fractions (25 mL) containing the product were identified by TLC analysis (4:1 hexane:
ethyl acetate as eluent,
Rf = 0.41) (Figure 3P). Fractions 41-74 containing the pure desired product were collected while fractions 76-80 contained products with other impurities. Test tubes containing the pure desired product were each rinsed with
EtOAc (2 x 3 mL), and the rinses were transferred into the collection flask.
46.
N'-(3-(Cyclopentyl(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)methyl)-3-phenylcyclobutylidene)-2,4,6-trimethylbenzenesulfonohydrazide (
3):
Note: The Z/E mixture (ratio: 2/1) was observed via 1H NMR and NMR characterization for the mixture was given. mp >200 ℃
1H NMR
pdf (500 MHz, acetone-d
6) δ: 9.22 - 8.94 (m, 1H), 7.57 - 7.23 (m, 4H), 7.18 (ddt,
J = 7.5, 5.2, 1.5 Hz, 1H), 6.98 (s, 2H), 3.50 - 3.35 (m, 1H), 3.33 - 3.03 (m, 3H), 2.62 (s, 3H), 2.61 (s, 3H), 2.26 (d,
J = 1.9 Hz, 3H), 1.61 - 1.43 (m, 6H), 1.37 - 1.23 (m, 2H), 1.19 - 1.16 (m, 13H), 1.12 - 1.04 (m, 1H).
13C NMR
pdf (151 MHz, acetone-d
6) δ: 155.7, 155.6, 148.9, 148.9, 143.1, 143.1, 140.8, 140.8, 134.8, 134.8, 132.5, 128.7, 128.7, 127.9, 127.9, 126.7, 84.0, 84.0, 48.4, 47.1, 44.8, 43.6, 43.4, 43.4, 40.4, 40.3, 34.7, 34.6, 31.6, 31.5, l25.9, 25.9, 25.5, 25.4, 25.4, 25.4, 25.2, 23.4, 23.4, 20.9;
. 11B NMR
pdf (128 MHz, CDCl
3) δ: 34.83; HRMS (ESI+) calc. for C
31H
43BN
2O
4S [M+H]
+ 551.3109, found 551.3101.
47. The purity of
3 was determined to be 97.9 wt% by qNMR
pdf using
1,3,5-trimethoxybenzene (Sigma-Aldrich, >99%) as the external standard.
48. A second run performed at full scale provided 2.76 g (42%) of
3 at 97.7% purity.
49.
Dioxane (99.5%, extra dry with molecular sieves, stabilized) was purchased from Acros Organics and used after it was received.
50. An aliquot (0.1 mL) used for TLC monitoring was removed and diluted with 0.9 mL
diethyl ether, and reaction progress was determined by TLC analysis on silica gel with 1:3
EtOAc: hexanes as eluent and visualization with CAM stain (Figure 4C,
Rf = 0.57).
51. Fractions (25 mL) containing the product were identified by TLC analysis (10:1 hexane:
diethyl ether as eluent,
Rf = 0.57). Fractions 82-126 mainly contained the desired product. For fraction 96-126, pure products are contained. Fractions 82-94 contained the product with the side-pro which could be purified via another column chromatography. Test tubes containing the desired product were each rinsed with Et
2O (2 x 3 mL), and the rinses were transferred into the collection flask.
52.
2-(2-Cyclopentyl-3-phenylbicyclo[1.1.1]pentan-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (
4): mp 41-43 ℃;
1H NMR
pdf (600 MHz, CDCl
3) δ: 7.30 - 7.23 (m, 2H), 7.22 - 7.13 (m, 3H), 2.75 (dd,
J = 9.8, 2.8 Hz, 1H), 2.38 - 2.25 (m, 2H), 2.08 - 2.01 (m, 2H), 1.98 (d,
J = 1.6 Hz, 1H), 1.82 (ddt,
J = 11.6, 7.2, 3.9 Hz, 1H), 1.65 - 1.37 (m, 5H), 1.279 (s, 6H), 1.276 (s, 6H), 1.25 - 1.13 (m, 1H), 0.96 - 0.86 (m, 1H) ppm;
13C NMR
pdf (151 MHz, CDCl
3) δ: 141.7, 128.0, 126.2 (2C), 83.4, 73.4, 52.8, 50.1, 45.5, 37.1, 32.7, 32.2, 25.8, 25.2, 25.0, 24.9 ppm;
11B NMR
pdf (128 MHz, CDCl
3) δ: 31.74 ppm; HRMS (ESI+) calc. for C
22H
31NO
2 [M+H]
+ 339.2490, found 339.2492.
53. The purity of
4 was determined to be 97.7 wt% by qNMR
pdf using
1,3,5-trimethoxybenzene (Sigma-Aldrich, >99%) as the external standard.
54. The compound
4 could be converted into colorless crystal from its saturated solution in CH
2Cl
2. The X-ray crystallographic coordinates for compounds
4 have been deposited at the Cambridge Crystallographic Data Center (CCDC) with accession code 2062924.
55. A second run performed at full scale provided 2.31 g (76%) of
4 at 97.2% purity.
3. Discussion
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved