1. Procedure (Note 1)
A. 2,5-Dibromohexanedioyl dichloride (1). A one-necked, 250 mL round-bottomed flask equipped with a Teflon-coated, egg-shaped magnetic stir bar (30 x 10 mm) is connected to jacketed and coiled condenser (250 mm, Note 2). The top joint is fitted with a vacuum adapter that is connected to a Drechsel bottle via Teflon tubing. Teflon sleeves are added to both connections to prevent freezing of the joints. The Drechsel bottle head is connected via Teflon tubing to a glass funnel (diameter: 75 mm) that is positioned over a 1 M sodium hydroxide solution (200 mL) in a 500 mL beaker (Figure 1A) (Note 3). The reaction flask is sequentially charged with adipic acid (10.0 g, 68.4 mmol, 1.0 equiv) (Note 4) and thionyl chloride (30 mL, 413 mmol, 3 equiv) (Note 5) by temporarily removing the condenser. The reaction flask is lowered into an oil bath that is preheated to 90 ℃ (Note 6), and the reaction mixture is refluxed for 90 min with stirring (400 rpm). At the completion of the reflux, the colorless suspension is a pale-yellow solution (Notes 7 and 8) (Figure 1B), and the funnel becomes white over time (Figure 1C).
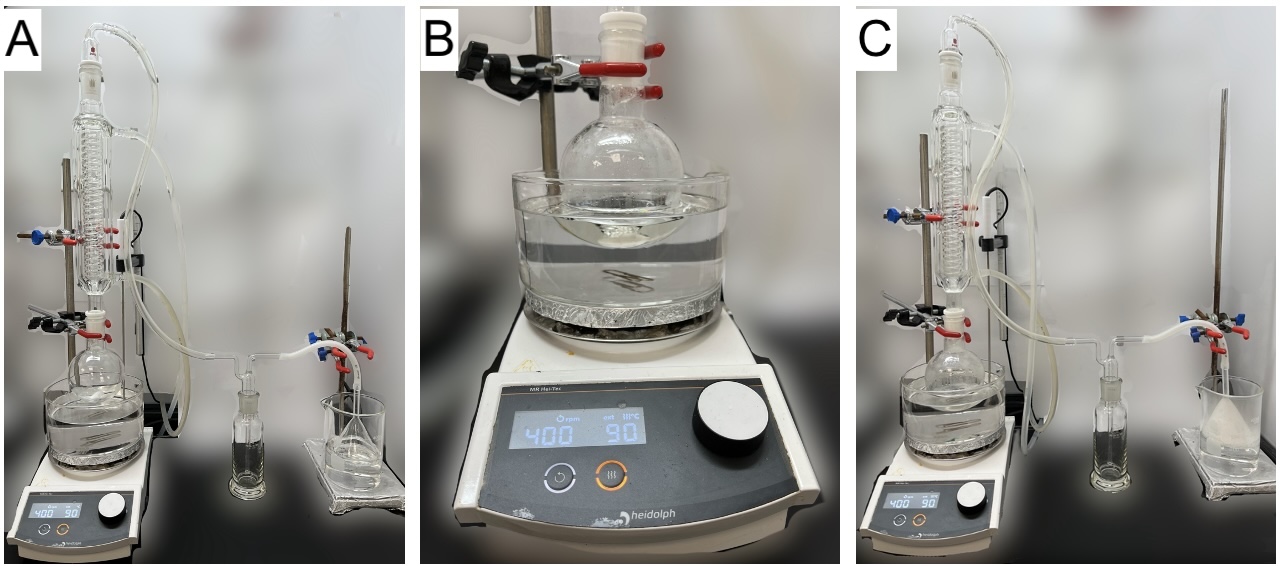
Figure 1. (A) Assembled reaction apparatus; (B) Reaction flask after completion of reaction; (C) Reaction apparatus after reflux (photos provided by checkers)
The heater is switched off, and the reaction mixture is allowed to stir for 10 min, over which time the temperature of the oil bath decreases from 83 ℃ to 47 ℃. The beaker containing the sodium hydroxide solution is replaced by a fresh 500 mL beaker containing 1.0 M sodium thiosulfate solution (Note 9). The condenser is temporarily removed. Cyclohexane (40 mL) (Note 10), N-bromosuccinimide (29.2 g, 164 mmol, 1.2 equiv) (Note 11) and hydrobromic acid (40 μL, 9 M, 360 μmol, 0.5 mol %) (Note 12) are sequentially added with stirring. The condenser is replaced. The reaction mixture turns pale orange upon the addition of N-bromosuccinimide, and the color deepens significantly when the hydrobromic acid is added (Figure 2A). The reaction mixture is heated to reflux for 2.5 h in a 90 ℃ oil bath, during which time the deep orange suspension and orange vapor (Figure 2B) first turn into a muddy yellow suspension with a colorless vapor (Figure 2C), and eventually to a black suspension with colorless vapor (Notes 13 and 14) (Figure 2D). The reaction mixture is then cooled in an ice-water bath for 30 min. The reaction mixture is filtered through a sintered glass Büchner funnel (Note 15) into a 250 mL round-bottomed flask under house vacuum (~75 mm Hg) (Figure 3). The filter cake is washed with methyl tert-butyl ether (MTBE) (3 x 20 mL) (Notes 16 and 17). The black filtrate is concentrated by rotary evaporation (40 ℃, 30 mmHg) to provide 1 as a black, viscous oil. The product is immediately carried on to the next step of the reaction sequence.
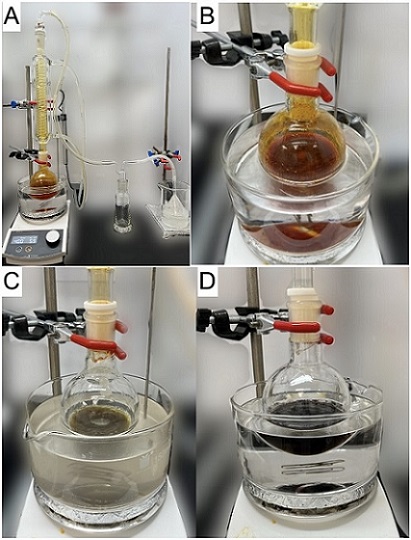
Figure 2. (A) Set-up of the bromination reaction; (B) Orange-red appearance at beginning of the reaction; (C) Muddy yellow suspension with colorless vapor; (D) Black suspension upon completion of the reaction (photos provided by checkers)
Figure 3. Filtration of solids (photo provided by checkers)
B. 2,5-Dibromohexanediamide (DBHDA) (2). A three-necked 250 mL round-bottomed flask (Note 18) equipped with a Teflon-coated, egg-shaped magnetic stirrer bar (30 x 10 mm) is fitted with a thermometer adapter and a thermometer on a side neck, and with a 150 mL pressure-equalizing addition funnel in the middle neck. The third neck is left open (Figure 4) (Note 19). Compound 1 (Note 20) is transferred to the addition funnel (Note 21), and the funnel is capped with a stopper. Ice-cold ammonium hydroxide solution (80 mL, 13.2 M (aq), 1.0 mol, 7.7 equiv) (Note 22) is poured into the reaction flask. The flask is cooled in an ice water-sodium chloride bath (-5 ℃) for 5 min. The black viscous oil (1) is added dropwise with rapid stirring (400 rpm) over 1 h, at a rate that maintains the internal temperature below 15 ℃. The addition of the oil causes the reaction mixture to immediately turn into a vivid blue color (Figure 5A), which becomes more intense as more compound 1 is added. An off-white precipitate forms over time. After the addition is complete, stirring is continued for one more hour in an ice-water bath (Note 23). After 1 h, the reaction mixture appears as green solids suspended in a brown/black liquid (Figure 5B). The green solids are collected using a Büchner funnel (Note 24) under house vacuum (~75 mmHg). The reaction vessel is washed twice with the filtrate, which is again passed through the filter.

Figure 4. Assembled reaction set up for Step B (photo provided by checkers)
Figure 5. (A) Blue mixture when 1 is added to ammonium hydroxide solution; (B) Appearance of reaction mixture upon completion (photos provided by authors)
The crude green powder (~28 g when damp) (Figure 7A) is transferred to a single-necked 250 mL round-bottomed flask equipped with a Teflon-coated egg-shaped magnetic stir bar (30 x 10 mm). The solids are triturated by stirring in 50% aqueous methanol (100 mL) (Note 25) for 30 min while the flask is heated using an aluminum heating block (Note 26) (Figure 6) that is preheated to 60 ℃. The mixture is cooled at room temperature (~20 ℃) for 10 min to approximately 30 ℃ (Notes 27 and 28). The solids are isolated by filtration using a Büchner funnel under house vacuum (~75 mmHg) (Note 29), washing out the reaction vessel once with the brown filtrate. The off-white powder thus obtained (Figure 7B) is transferred to a single-necked 250 mL round-bottomed flask equipped with a Teflon-coated egg-shaped magnetic stir bar (30 x 10 mm) and triturated again by stirring in 50 % aqueous methanol (100 mL) (Note 25) for 10 min while the flask is heated using an aluminum heating block preheated to 60 ℃ (Note 30). The mixture is cooled for 30 min at room temperature (~20 ℃). The solids are isolated by filtration using a Büchner funnel under house vacuum (~75 mm Hg) (Note 29), washing out the reaction vessel once with the yellow filtrate. The filter cake (Figure 7C) is dried in a vacuum desiccator (<1 mm Hg,) (Note 31) overnight over anhydrous calcium chloride (Note 32). The off-white powder is crushed with a spatula and then dried for 3 h in a vacuum desiccator to yield 2 as a microcrystalline, off-white powder (5.82 g, 19.3 mmol, 28%) (Notes 33, 34, and 35) (Figure 7D).

Figure 6. Reaction set-up for trituration (photo provided by checkers)
Figure 7. (A) Crude DBHDA (2) before trituration; (B) After first trituration; (C) After second trituration; (D) After drying (photos provided by authors)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
adipic acid,
thionyl chloride,
N-bromosuccinimide,
hydrobromic acid,
diethyl ether,
ammonium hydroxide,
cyclohexane and
methanol. Proper fume hood operation should be checked prior to use.
2. The checkers used a Synthware condenser that is jacketed and has an internal coil, Part no. C334250. The authors used a Davis condenser.
3. The
sodium hydroxide solution was prepared by dissolving
sodium hydroxide (8.0 g, AR grade, Dieckmann (Hong Kong) Chemical Industry Co. Ltd), in water (200 mL).
4.
Adipic acid was purchased from Scientific Laboratory Supplies Ltd (99 %) and used as received. The checkers purchased
adipic acid (> 99.0%) from TCI, which was used as received.
5.
Thionyl chloride was purchased from Acros Organics (99.5 %) and used as received. The checkers purchased
thionyl chloride (99%) from Energy Chemical, which was used as received.
Thionyl chloride was transferred to the reaction flask using a plastic syringe. An excess of
thionyl chloride is used because it acts as both dehydrating agent and the reaction solvent.
N-bromosuccinimide is nearly insoluble in
cyclohexane, so the small amount of
thionyl chloride acts as a co-solvent and allows the reaction to proceed more readily.
6. The oil bath was heated and stirred using a Heidolph Hei-Tec Magnetic Heater-stirrer, equipped with a temperature probe.
7. Reaction progress can be monitored by TLC. The checkers used 50% v/v
ethyl acetate/
hexane as the mobile phase and visualized the TLC plate using bromocresol green stain (Figure 8B). The stain was prepared by dissolving 0.04 g bromocresol green in 100 mL of absolute
ethanol, then adding 0.1 M aqueous
NaOH dropwise while swirling until the colorless solution just turns blue.
Figure 8 -TLC of the chlorination of adipic acid (Note 7); (Eluent: 50% v/v ethyl acetate/hexane) SM: starting material (adipic acid, Rf 0.22); Co: co-spot; R: reaction mixture, adipoyl chloride (Rf 0.65) (photo provided by checkers)
8.
Adipoyl chloride can be isolated by removing the volatiles by rotary evaporation (50 ℃, 75 mmHg) to yield a colorless oil, which can be distilled at 160 ℃ at 14 mmHg.
9. 1 M
Sodium thiosulfate solution was prepared by dissolving 31.6 g
Sodium thiosulfate (96.0 %, Bide Pharmatech Ltd.) in water (200 mL).
10.
Cyclohexane was purchased from Sigma Aldrich (99.8 %) and used as received. The checkers purchased
cyclohexane (99.5 %) from Duksan Pure Chemicals Ltd., which was used as received.
11. The authors purchased
N-bromosuccinimide from Alfa Aesar (99 %); the checkers purchased
N-bromosuccinimide (99 %) from Thermo Scientific.
N-Bromosuccinimide was recrystallized using the following protocol: In a 2 L conical flask open to air, water (600 mL) is heated to 90 ℃ on a hotplate with rapid stirring provided by a magnetic stir bar.
N-Bromosuccinimide (60.0 g) is added and stirred at the same temperature until homogeneous. The stir bar is removed, and the bright orange solution is cooled in a refrigerator (4 ℃) for at least 3 h, but ideally overnight. The colorless plates are collected by vacuum filtration using filter paper on a 150 mL Büchner funnel, then dried overnight in a vacuum desiccator containing phosphorus pentoxide, to yield
N-bromosuccinimide as colorless plates (53.4 g). Use of less pure
N-bromosuccinimide in the procedure leads to greater contamination with mono-brominated products; use of greater than 1.2 equiv of
N-bromosuccinimide induces a greater conversion to the dibrominated product but complicates the purification, which in turn results in a lower isolated yield of
1.
12.
Hydrobromic acid was purchased from Fluka (8.89 M) and used as received. The checkers purchased
hydrobromic acid (48 wt % in water) from Shanghai Aladdin Bio-Chem Technology Co. Ltd, which was used as received. The reaction proceeds without this catalyst; however, the rate is decreased significantly.
13. The reaction is complete when it reaches the pale-yellow stage, typically after 1.5 to 2.5 h. No significant change in yield is observed when the reaction is allowed to run for a longer time.
14. The reaction progress can be monitored by TLC using the following protocol: An aliquot (100 μL) of the reaction mixture is withdrawn using a microliter syringe and mixed with 500 μL of
isopropanol, then allowed to stand for 2 min. The diluted reaction mixture is spotted on a silica/aluminum-backed TLC plate and run against a standard prepared from the first phase of the reaction (from
Note 7), in a mobile phase made by mixing chloroform (10 mL) with formic acid (100 μL). The plate is dried then visualized using
phosphomolybdic acid stain (Figure 9).
Figure 9 - TLC of the bromination of adipoyl chloride (Note 14); Starting material (SM), Co: co-spot, and reaction mixture (R) (photo provided by checkers)
15. The authors filtered the reaction mixture through a sintered glass Hirsch funnel into a 250 mL round-bottomed flask (B19, FR250/2S) with a side arm adapter (MF/18/2/3C) under a mild vacuum. The checkers filtered the reaction mixture through a 60 mL Büchner funnel (Figure 3) with a medium porosity frit and a sidearm for connecting to house vacuum (~75 mm Hg).
16.
Methyl tert-butyl ether (
MTBE) was purchased from Alfa Aesar (99 %) and used as received. The checkers purchased
MTBE (99.5 %) from RCI Labscan Ltd., which was used as received.
Diethyl ether can be used instead of
MTBE in this step.
17. Approximately 9 g of succinimide is isolated.
18. The use of a two-necked round-bottomed flask causes significant buildup of ammonia vapors, which react with the acid chloride to form impressive stalactites as shown in Figure 10. Stalactites form from ammonia gas in the head space above the reaction mixture reacting with the liquid acid chloride, which forms a thin solid layer on the outside of the liquid. As the reaction proceeds under these conditions, the eventual collapse of the stalactites into the reaction mixture causes a 'spike' in reaction temperature. It is of particular importance to avoid this when the reaction is performed on larger scales. A photograph of a stalactite can be seen in Figure 10.
Figure 10. Stalactite of acid chloride which forms when ammonia vapor is allowed to build up (photo provided by authors)
19. During the reaction, the ammonium chloride vapors cloud the headspace inside the flask; inert gas such as
nitrogen can be blown through this open neck to clear the headspace so that the thermometer can be read.
20. Attempts were made to distill
1, but it was found to be too unstable to purify.
21. The viscous black oil is poured directly into the addition funnel with no washing being performed; the marginal decrease in yield is offset by the convenience of the product precipitating from the reaction.
22.
Ammonium hydroxide solution (18.8 M) was purchased from Fisher Scientific UK, diluted to 13.4 M using Milli-Q ultrapure water, and stored at 4 ℃ prior to use. The checkers purchased
ammonium hydroxide solution (25% in water, 99 % purity) from Thermo Scientific, which was used as received.
Ammonium hydroxide is used in excess to provide a medium for reaction and as a method of capturing released
hydrogen chloride. A high concentration of
ammonium hydroxide is preferred as this favors amide formation over hydrolysis, and a larger volume acts as a better heat sink for this strongly exothermic process.
23. Monitoring the reaction of
1 with ammonia is complicated by product precipitation and low solubility in many common organic solvents. The reaction between the concentrated ammonia and
1 is assumed to be rapid; allowing this reaction to proceed for a longer time does not lead to an increase in yield.
24. The authors used a sintered glass Büchner funnel (55 mm) for the isolation. The checkers used a 150 mL Büchner funnel, fitted with filter paper and a sidearm to connect to the house vacuum.
25.
Methanol was purchased from Honeywell (99.9 %) and diluted to 50 % using Milli-Q ultrapure water and stored in a refrigerator (4 ℃) prior to use. The checkers purchased
methanol from Scharlau Science Group SL (99.9%), which was diluted to 50% using tap water.
26. The authors used a preheated oil bath. The checkers used a preheated aluminum heating block with a well for a 250 mL round-bottomed flask, which keeps the flask surface clean.
27. The checkers found that it was imperative to cool the hot mixture prior to filtration to collect
2. If the trituration mixture was hot-filtered at 60 ℃, only the
meso-diastereomer of
2 (
Note 28) was collected, effectively resulting in a halving of the final yield of
2. The DL-diastereomer of
2 appears to be more soluble and remains in solution.
28. The physiochemical properties of
meso-
2 are as follows:
2 mp > 200 ℃ (d); R
f (
EtOAc) = 0.16;
1H NMR
pdf (400 MHz, d
6-DMSO) δ: 7.67 (s, 2H, NH
2), 7.29 (s, 2H, NH
2), 4.33 (m, 2H, CBrH), 2.04 (m, 2H, CH
2), 1.82 (m, 2H, CH
2);
13C NMR
pdf (101 MHz, d
6-DMSO) δ: 169.7 (C=O), 48.2 (CBr), 32.6 (CH
2); IR (ATR) 3340, 3172, 1665, 1443, 1322, 1251, 1195, 820, 802, 769, 637, 613 cm
-1; HRMS (ESI
+) (M
79Br
79Br+H
+) Calcd: 300.9182 (51.2%), Found: 300.9186 (50.4 %); (M
79Br
81Br+H
+) Calcd: 302.9161 (100
%), Found
: 302.9164 (100%); (M
81Br
81Br + H
+) Calcd: 304.9142 (49.1 %) Found: 304.9144 (47.3%).
29. The authors filtered the solids through a sintered glass funnel (55 mm). The checkers used a 150 mL Büchner funnel (60 mm diameter) fitted with a filter paper.
30. The authors triturated the solids for 30 min before isolation of the solids.
31. TLC analysis can be performed on both the solids and mother liquors during purification. The solids are dissolved in DMF, while the mother-liquors can be spotted neat then gently evaporated. Analysis by TLC was performed by using a mobile phase prepared from chloroform (8 mL), formic acid (100 μL) and
methanol (2 mL). Ninhydrin stain, prepared by dissolving ninhydrin (0.1 g) in acetic acid (0.5 mL) and acetone (100 mL), was used for visualization (Figure 11).
Figure 11. TLC analysis of DBHDA purification (Note 31) showing TLC showing ML (mother liquor); 1T: solid obtained from 1st trituration; 2T: solid obtained from 2nd trituration. 2 (Rf = 0.43): brown spot; monobrominated bis(amide) (Rf = 0.30): brown spot (photo of C provided by checkers)
32. Anhydrous
calcium chloride was purchased from Honeywell Fluka (93 %) and used as received. The checkers purchased anhydrous
calcium chloride from Unichem Specialty Chemicals LLC (96.0%), which was used as received.
33. The physiochemical properties of
2 (
meso and DL-diastereomers are in essentially equal abundance) are as follows: mp > 200 ℃ (d); R
f (
EtOAc) = 0.16;
1H NMR
pdf (400 MHz, d
6-DMSO) δ: 7.68 (s, 2H, NH
2), 7.30 (s, 2H, NH
2), 4.33 (td,
J = 4.24, 1.43 Hz, 2H, CBrH) and 2.06 - 1.82 (m, 4H, CH
2);
13C NMR
pdf (101 MHz, d
6-DMSO) δ: 169.9 (C=O), 169.8 (C=O), 48.5 (CBr), 48.2 (CBr), 32.6 (CH
2) and 32.5 (CH
2); IR (ATR) 3339, 3171, 1663, 1422, 1322, 1277, 1251, 1223, 1195, 820, 801, 769, 661, 612 cm
-1; HRMS (ESI
+) (M
79Br
79Br+H
+) Calcd: 300.9182 (51.2%), Found: 300.9173 (50.4 %); (M
79Br
81Br+H
+) Calcd: 302.9161 (100%), Found: 302.9154 (100%); (M
81Br
81Br + H
+) Calcd: 304.9142 (49.1 %) Found: 304.9135 (47.3%);
34. The checkers determined the purity of compound
2 (
syn- and
anti-diastereomers) to be 98.2 % ± 0.65, by two repeats of quantitative
13C NMR
pdf spectroscopy against an internal standard of ethylene carbonate (72.2, 73.5 mg of
2 and 19.1, 19.1 mg of internal standard respectively) in d
6-DMSO (600 μL). The purity was found to be 97.5 % using quantitative
1H NMR
pdf spectroscopy against an internal standard of
1,3,5-trimethoxybenzene (26.6 mg of
2, 18.1 mg of internal standard respectively) in
d6-DMSO (600 μL). The checkers purchased ethylene carbonate from TCI (> 99%) and
1,3,5-trimethoxybenzene (99.99%) from Sigma Aldrich which were used as received.
35. The procedure was repeated on half-scale by the checkers, with a yield of 3.01 g (29%) of
2, with purity of 98.0%.
3. Discussion
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved