Org. Synth. 2024, 101, 309-326
DOI: 10.15227/orgsyn.101.0309
Synthesis of Benzyl-δ-Truxinate via Enantioselective [2+2] Photocycloaddition
Submitted by Rodrigo Villanueva, Ellie F. Plachinski, and Tehshik P. Yoon*
Checked by Yu-Che Chang and M. Kevin Brown
1. Procedure (Note 1)
A. (E)-1-(1-Methyl-1H-imidazol-2-yl)-3-phenylprop-2-en-1-one (1). A 100 mL round-bottomed flask (24/40 joint) is equipped with a 25.4x8mm Teflon-coated magnetic stir bar and charged with 1-(1-methyl-1H-imidazol-2yl)ethan-1-one (2.35 g, 18.9 mmol, 1.00 equiv.) (Note 2) and potassium hydroxide (0.370 g, 6.62 mmol, 0.350 equiv.) (Note 3). To the solids are added benzaldehyde (2.01 g, 18.9 mmol, 1.00 equiv.) and EtOH/H2O (1:1; v/v, 34 mL) (Note 4) before the flask is sealed with a rubber septum. The edges of the septum are sealed with electrical tape before inlet, and outlet needles are pierced through the septum. The inlet needle is connected to a Schlenk line under positive nitrogen pressure, and the solution is sparged with nitrogen for 10 min while stirring at 240 RPM, at which point the yellow solution becomes heterogeneous (Figure 1). The inlet and outlet needles are then removed before sealing the punctures with electrical tape and covering the flask with aluminum foil (Note 6).

Figure 1. a) Solution of KOH, benzaldehyde, and N-acyl-imidazole in EtOH/H2O); b) After 10-minute sparge while stirring (photos provided by submitters)
After stirring for 21 h at room temperature (22 ℃), the septum and stir bar are removed from the flask. The product is collected from the off-white heterogenous mixture via vacuum filtration over a 60 mL glass Buchner funnel (fine porosity), washing the flask with EtOH/H2O (2:1; v/v, 5 mL) and collecting the filtrate in a 250 mL round-bottomed flask, attached by a glass vacuum filtration adapter (24/40 joints) (Figure 2a). The solid is dried on the frit for an additional 10 min before being transferred to a 40 mL scintillation vial and dried under high vacuum (<3 mmHg) for an additional 45 min to afford 3.56 g (89%) of (E)-1-(1-methyl-1H-imidazol-2-yl)-3-phenylprop-2-en-1-one (1) (Figure 2b) (Notes 6 and 7).
Figure 2. a) Vacuum filtration of crude reaction mixture through 60 mL glass Buchner funnel. b) Product 1 obtained as a pure white solid (photos provided by submitters)
B. (-)-Benzyl-δ-truxinate (2). A flame-dried 500 mL round-bottomed flask (24/40 joint) equipped with a 25.4x8mm Teflon-coated magnetic stir bar is charged with 1 (3.30 g, 15.5 mmol, 1.00 equiv.) and (S)-TRIP (2.46 g, 3.10 mmol, 0.20 equiv.) (Note 9) before sealing the flask with a rubber septum and piercing the septum with a needle. The flask is evacuated and backfilled with N2 three times over 25 min (2x5 min, 1x15 min). Meanwhile, a separate flame-dried 500 mL round-bottomed flask is charged with dry toluene (311 mL, measured in a flame-dried graduated cylinder under air) (Note 10) and sealed with a rubber septum. The solvent is sparged with nitrogen for 25 min. Both flasks are maintained under a positive pressure of nitrogen before transferring the sparged toluene to the reaction flask via cannula (Figure 3a). Upon completion of the cannula transfer, the nitrogen lines are removed, and the septum is sealed with electrical tape. The solution is sonicated for 3 min until homogeneous. The flask is placed in an EtOH bath maintained at 0 ℃ using an immersion cooler (Note 11). The reaction is stirred at 340 RPM and irradiated with two Kessil PR-160L 427 nm lamps (distance of 6 cm, 100% intensity) (Figure 3b). The apparatus is covered in aluminum foil during irradiation.
Figure 3. a) Cannula transfer of solvent to reagent-containing flask. b) Flask in Cryo-Cool with 2x 427nm Kessil Lamps pointed at the surface of the solution (photos provided by submitters)
After 45 h, the flask is removed from the cooling bath, allowed to warm to 23℃, and opened to air before being quenched with triethylamine (5 mL). The stir bar is removed, and the mixture is concentrated via rotary evaporation (50 ℃, 370 to 25 mmHg) (Figure 3c) to afford a brown viscous oil. The oil is reconstituted in DCM (10 mL) before being quantitatively transferred to a 100 mL round-bottomed flask (14/20 joint) and concentrated once more under identical conditions. To the flask is added a 25.4x8mm Teflon-coated magnetic stir bar, and benzyl alcohol (38 mL) is added via syringe before sonicating the solution for 5 min until homogeneous. The flask is sealed with a rubber septum, and a nitrogen inlet needle is used to sparge the solution for 12 min before sealing the holes with grease, Teflon tape, and electrical tape. The flask is placed in an oil bath before being brought to and maintained at 150 ℃ (Note 12) while stirring at 340 RPM. After 16 h, the flask is cooled to room temperature and opened to air before being fitted with a glass short-path distillation apparatus (14/20 joints) equipped with a thermometer and 100 mL collection flask (maintained at -78 ℃ in a bath of acetone and dry ice) (Figure 4c).

Figure 4. a) Solution after photochemical step; b) Concentrated and reconstituted in benzyl alcohol. c) Distillation apparatus; d) Resultant brown oil (photos provided by submitters)
All joints are sealed with a small amount of grease before they are fastened with Keck clips. Inlet and outlet hoses are attached to the condenser, and high vacuum is slowly applied (760 to 3 mmHg over 3 min) to the apparatus while still at room temperature. Once under vacuum, the flask is placed in an oil bath and heated to 95 ℃ over 45 min. When the oil bath reaches 65 ℃, a small sheet of aluminum foil is placed over the distillation neck. The temperature of the oil bath is maintained at 95 ℃ to remove the excess benzyl alcohol. Once completed, the apparatus is cooled to 23 ℃, and the brown oily residue (Figure 4d) is taken up in DCM (30 mL) (Note 13) before being quantitatively transferred to a 100 mL round-bottomed flask. At this point, the crude reaction is visualized by TLC (note 14) (Figure 5).
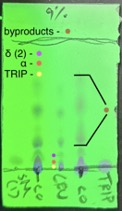
Figure 5. After removal of benzyl alcohol, the crude reaction is analyzed by TLC on silica gel using 9% acetone in hexanes as eluent. Spots are visualized by UV light (254 nm). Note: SM (1) = Starting Material (1), CRU = crude reaction mixture, TRIP, co = co-spot lanes. The desired δ-truxinate (2) (Rf=0.11) elutes after byproducts (Rf=0.21-0.61) and before undesired α-truxillate (Rf=0.07) and TRIP (Rf=0.03). The photo was provided by the authors.
The solution is then concentrated onto Celite (6.8 g) (Figure 6a) (Note 14) via rotary evaporation (30 ℃, 370 to 25 mmHg) before being loaded on a 410 mL (or 205 g) silica flash column (Figure 6b) (Note 15). 1.5 cm of sand is carefully charged over the dry loaded celite, and the column is eluted with a gradient of acetone in hexanes (5% to 9%) . A series of bright yellow impurities elutes immediately before the product and can be visually monitored on the column (Figure 6c).
Figure 6. a) Crude mixture over 6.8 g of Celite (dryload); b) Dryload over 205 g packed silica gel and under 1.5 cm of sand c) Elution of bright yellow impurities d) Product 2 obtained as an amorphous light-yellow solid after column chromatography (photos provided by submitters)
Product-containing fractions are identified via TLC analysis on silica gel with 9% acetone in hexanes (Note 16), transferred to a 1 L round-bottomed flask (rinsing once with 2 mL DCM), and concentrated via rotary evaporation (30 ℃, 370 to 25 mmHg) to afford a yellow oil. The oil is transferred to a tared 40 mL scintillation vial using ethyl acetate (5 mL) before concentrating once more. The resultant oil is then placed under high vacuum (<3 mmHg) for 50 min, affording 2.15 g (58%) of pure (-)-benzyl-δ-truxinate (2) as a yellow solid (Figure 6d) (Note 17).
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
benzaldehyde,
1-(1-methyl-1H-imidazol-2yl)ethan-1-one,
reagent alcohol,
KOH,
toluene,
(R)-TRIP,
benzyl alcohol, as well as the proper procedures for high temperature reactions, and reactions invoking near-UV light. The photochemical set up should be rigorously covered in aluminum foil to avoid inadvertent light exposure.
2.
1-(1-Methyl-1H-imidazol-2yl)ethan-1-one (>95%) is available from AbovChem but can be prepared via the following procedure: under
nitrogen atmosphere, a sealed round-bottomed flask is charged with
N-methyl-imidazole (1.1 equiv.) and
THF before
n-butyl lithium (1.1 equiv., 2.5 M in hexanes) is added via syringe at 0 ℃ and allowed to stir for 15 min. A separate round-bottomed is charged with N-acetyl-morpholine and
THF (0.65 M total) under
nitrogen atmosphere at -78 ℃. The solution of lithiated imidazole is added via cannula transfer over 5-10 min, and the resultant mixture is stirred for 30 min at -78 ℃ before being let warm to 23 ℃ over 30 min. The product is isolated via flash column chromatography eluting with 35%
acetone in hexanes (R
f=0.27) (86%).
3.
Potassium hydroxide (
KOH) (>85%, pellets) was purchased from Sigma Aldrich and used as received.
4.
Benzaldehyde (>99%) was purchased from Sigma Aldrich and used as received.
5.
Ethanol (
EtOH, 200 proof) was purchased from Decon and used as received. Water used in the reaction was deionized but not degassed.
6. Aluminum foil is used as a precautionary measure. The product of the reaction (
1) is photosensitive and prone to visible-light mediated dimerization over time; it should be stored in the dark.
7.
(E)-1-(1-Methyl-1H-imidazol-2-yl)-3-phenylprop-2-en-1-one (
1):
1H NMR
pdf (500 MHz, CDCl
3) δ 8.08 (d, J = 16.0 Hz, 1H), 7.83 (d, J = 16.0 Hz, 1H), 7.73 - 7.66 (m, 2H), 7.40 (dd, J = 5.0, 2.0 Hz, 3H), 7.22 (s, 1H), 7.08 (s, 1H), 4.10 (s, 3H).
13C NMR
pdf (126 MHz, CDCl
3) δ 180.7, 144.2, 143.6, 135.1, 130.6, 129.5, 129.0, 128.9, 127.4, 122.9, 36.5. HRMS (ESI)
m/z: Calcd. for C
13H
13N
2O [M+H]: 213.1022, found: 213.1015. The purity of 1 was determined to be 98.6% by qNMR
pdf using
1,3,5-trimethoxybenzene (purity >99%, purchased from Sigma Aldrich, used as received) as an internal standard.
8. A second full scale reaction provided the desired product in 83% yield.
9.
(S)-TRIP (98%,
(S)-3,3'-bis(2,4,6-triisopropylphenyl)-1,1'-binaphthyl-2,2'-diyl hydrogenphosphate) was purchased from Ambeed and used as received. The authors' procedure utilized
(R)-TRIP as a catalyst in the reaction which provided the enantiomer of
2.
10. Anhydrous
toluene was purchased from Sigma Aldrich (>99.9%, HPLC grade), dried by mBraun SPS, and stored under Argon.
11. The
EtOH bath temperature is controlled using a Thermo Fischer EK90 immersion cooler. The
EtOH bath should be refilled after 30 h.
12. Oil bath temperature was maintained by an IKA-CMAG HS7 stir plate and temperature probe.
13.
Dichloromethane (
DCM) (>99.5%, reagent grade) was purchased from Sigma Aldrich and used as received.
14. Diatomaceous earth (Celite 545) was purchased from Fisher Scientific and used as received.
15. Silica Gel (SiO
2) was purchased from DisiDry-SilicaGel and was used as received. For flash column chromatography, a slurry of 410 mL of silica gel in 5%
acetone in hexanes (500 mL) is loaded onto a 1.0 L glass column (12" [L], 3" [W], 24/40 joint) over 2 cm of sand. Once packed, the dryload is evenly distributed above the surface of the silica, after which an additional 1.5 cm of sand is added on top. After full adsorption, the column is eluted with 5%
acetone in hexanes (900 mL, 2.2 CV), 7%
acetone in hexanes (700 mL, 1.7 CV), and 9%
acetone in hexanes (600 mL, 1.45 CV).
16. Including the eluent used to slurry the dry silica, the first 850 mL (or 40 16x150 mm fraction volumes) of eluent can be discarded before collecting 40 16x150 mm fractions. Fractions are checked via TLC using 9%
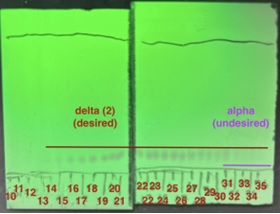
acetone in hexanes as eluent and visualizing with 254 nm UV light. The product is observed in fractions 14-35 and is collected from 14-30 (fractions 31-35 show significant coelution with the undesired α-truxillate). (photos provided by submitters)
17.
(-)-Benzyl-δ-truxinate:
1H NMR
pdf (500 MHz, CDCl
3) δ 7.38 - 7.21 (m, 20H), 5.25 - 5.10 (m, 4H), 3.83 - 3.75 (m, 2H), 3.57 - 3.49 (m, 2H).
13C NMR
pdf (126 MHz, CDCl
3) δ 172.4, 141.0, 135.9, 128.8, 128.7, 128.3, 128.1, 127.3, 127.0, 66.8, 47.1, 45.0. HRMS (ESI)
m/z: Calcd. for C
32H
28O
4Na [M+Na]
+: 499.1880, found: 499.1871. The purity of (2) was determined to be 97.3% by qNMR
pdf using
1,3,5-Trimethoxybenzene (purity > 99%, purchased from Sigma Aldrich, used as received) as internal standard. The enantiomeric excess was determined to be 93% by chiral HPLC
pdf on a Phenomenex Lux 3u Cellulose-2 column over 25-minute of 10%
isopropanol/hexanes at a flow rate of 1 mL/minute (monitoring at 254 nm). The retention time of the major enantiomer was 13.93 min., and the retention time of the minor enantiomer was 7.26 min.
18. Racemic (2) can be prepared using (+/-)-TRIP in an analogous procedure, or by the following: a flame dried 4 mL vial equipped with a magnetic stir bar is charged with
(E)-1-(1-methyl-1H-imidazol-2-yl)-3-phenylprop-2-en-1-one (
1) (30.0 mg, 0.14 mmol, 1.0 equiv). The vial is evacuated and backfilled with N
2 three times over 15 min (2x2.5 min, 1x10 min) before being charged with dry
DCM (2.8 mL, 50 mM). The vial is stirred (340 RPM) and irradiated with a Kessil PR-160L 390 nm lamp (distance of 5 cm, 100% intensity) at 23 ℃. After 16 h, the solution is concentrated via rotary evaporation. The remaining oil is taken up in BnOH (0.35 mL, 0.2M) before the vial is sealed and placed in a 150 ℃ oil bath while stirring at 500 RPM. After 18 h, the
benzyl alcohol is removed via vacuum distillation (90 ℃) and the remaining oil is purified by preparatory TLC (3:1 hexanes/
ethyl acetate, R
f=0.2) to afford the desired product as an off-white amorphous solid (12.0 mg, 36% yield).
pdf
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The truxinates are a large class of natural products comprising over 100 dimeric and pseudodimeric cyclobutanes that exhibit a wide range of biological activities.
2 Formally, these natural products arise from the [2+2] cycloaddition of variously substituted cinnamic acid derivatives.
3 In principle, [2+2] photocycloaddition reactions would provide a uniquely direct approach towards the synthesis of the truxinate natural products that would construct two carbon-carbon bonds in a single step and set all four stereocenters about the cyclobutane core. Unfortunately, in general, predictable control over the regioselectivity, diastereoselectivity, and enantioselectivity of [2+2] photocycloadditions presents a formidable challenge, and due to the high energy of the electronically excited intermediates and the conventional stepwise mechanism of triplet-state cycloadditions, mixtures of products are commonly formed (Figure 7).
4Figure 7: Selectivity challenges in classical [2+2] photodimerizations
Recently, the Yoon lab developed a general platform for truxinate synthesis based upon asymmetric catalytic [2+2] photocyclodimerization of C-cinnamoyl imidazoles.
5 This study found that protonation of the Brønsted-basic imidazole by a chiral phosphoric acid (CPA) catalyst can result in a significant bathochromic shift. This chromophore activation
6 enables selective irradiation of the catalyst-bound substrate over the free unbound substrate, resulting in a highly enantioselective, diastereoselective, and regioselective photocycloaddition. The utility of this strategy was demonstrated through the syntheses of four dimeric truxinate natural products (Table 1).
Table 1. Selected Truxinate products via CPA induced chromophore activation
The optimal Brønsted acid catalyst that emerged from our investigations was BINOL-derived phosphoramide (3) (Figure 8a). The N-triflyl phosphoramide moiety is ideal for this application because its greater acidity compared to the parent phosphoric acid gives rise to a much larger red shift, which facilitates selective irradiation of bound substrate at low catalyst loadings. Nevertheless, we recognized that the accessibility of this method would be improved if a common commercial chiral phosphoric acid catalyst such as TRIP (4) could be used instead of 3. The present procedure is the result of a reoptimization campaign centered around the use of TRIP as a chiral Brønsted acid catalyst.
Figure 8. Comparison of (R)-TRIP with N-triflyl phosphoramidite and UV-Vis Absorption study of TRIP + Substrate
In agreement with our original studies, the (
R)-TRIP-bound substrate exhibits a much smaller red shift than (R)-NTPA-bound substrate (Figure 8b). The diminished bathochromic shift necessitated careful selection of irradiation wavelength and judicious temperature control to maximize enantioenrichment in the (
R)-TRIP-catalyzed reaction. Furthermore, the synthesis of the cinnamoyl-imidazole (1) involves a high-yielding aldol condensation from a commercially available methyl ketone. Finally, the key asymmetric [2+2] photocycloaddition and the cleavage of the imidazole auxillary can be conducted without chromatographic purification of the intermediate cycloadduct.
7 This sequence affords the desired enantioenriched truxinate building block with a convenient benzyl ester functional group.
In conclusion, we developed a facile three-step procedure for the preparation of benzyl-δ-truxinate on a 2 g scale. The enantioselective [2+2] photodimerization sets four stereocenters in a single step, and the final product can be carried forward through further functionalization.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
EtOH: Reagent Alcohol; (64-17-5)
1-(1-methyl-1H-imidazol-2yl)ethan-1-one; (85692-37-1)
Benzaldehyde; (100-52-7)
KOH: Potassium Hydroxide; (1310-58-3)
(E)-1-(1-methyl-1H-imidazol-2-yl)-3-phenylprop-2-en-1-one (1); (860772-43-6)
toluene; (108-88-3)
(R)-TRIP: (R)-3,3’-Bis(2,4,6-triisopropylphenyl)-1,1’-binaphthyl-2,2’-diylhydrogenphosphate;) (791616-63-2)
Benzyl alcohol; (100-51-6)
(+)-Benzyl-δ-truxinate: dibenzyl (1S,2S,3R,4R)-3,4-diphenylcyclobutane-1,2-dicarboxylate (2): (2149600-24-6)

|
Rodrigo Villanueva was born in Denton, Texas. He currently attends the University of Wisconsin-Madison and is expected to receive a BS in Chemistry in 2024. He joined the Yoon Group in 2022 where his work began on photochemical Copper Ligand-to-Metal Charge Transfer (LMCT) and later asymmetric [2+2] photocycloadditions. |

|
Ellie Plachinski obtained a B.S. in chemistry in 2020 from the Massachusetts Institute of Technology, where she conducted research in the laboratory of Prof. Alison Wendlandt. She is currently pursuing her Ph.D. in the Yoon Group in the Department of Chemistry at the University of Wisconsin-Madison. |

|
Tehshik Yoon is a Professor of Chemistry at the University of Wisconsin-Madison. He received his Ph.D. with David MacMillan at Caltech and perform postdoctoral research with Eric Jacobsen at Harvard. Since 2005, his research group has pioneered the development of visible light induced photochemical transformations. |

|
Yu-Che Chang was born and raised in Taiwan. He received his B.S. and M.S. from National Tsing Hua University in 2017 and 2019. He is currently a fourth-year graduate student in Prof. M. Kevin Brown's laboratory at Indiana University, Bloomington. His research mainly focuses on photochemical strain-release cycloadditions. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved