Org. Synth. 2025, 102, 276-302
DOI: 10.15227/orgsyn.102.0276
OS Techniques
Purification of Organic Compounds by Flash Column Chromatography
Submitted by Benjamin D. Senzer, Cholapat Varongchayakul, and Rick L. Danheiser*1
Brian Daniels and Vy M. Dong*2
Checked by Katerina Scotchburn and Sophie A. L. Rousseaux
1. Procedure (Note 1)
A. Purification of linalool by column chromatography with isocratic elution.
Dry-packing the column. A 55-mm diameter glass column (60 cm in length) with a 24/40 ground glass joint and Teflon stopcock is plugged at the bottom with cotton and secured by two clamps such that the column is perpendicular to the work surface (Figure 1). Sand (Note 2) is added to the column in an even layer until the curved portion at the bottom of the glass column is completely covered (Figure 2). Dry silica gel (84 g) (Note 3) is poured into the column via a powder funnel with care taken not to disturb the layer of sand. The height of the column of silica gel is 8 cm.
Figure 1. Glass chromatography column (photo provided by the authors)
Figure 2. (A) Cotton plug and sand at the bottom of the column; (B) Column packed with dry silica gel (photos provided by the authors)
Depositing the sample on Celite. A 200-mL, one-necked, recovery flask is charged with linalool (1.00 g, 6.48 mmol) (Note 4), linalyl acetate (0.10 g, 0.51 mmol) (Note 5), and farnesol (0.10 g, 0.45 mmol) (Note 6). Dichloromethane (15 mL) (Note 7) is added, and the mixture swirled to create a homogeneous solution (Notes 8, 9). Celite (2.5 g) (Note 10) is added, and the dichloromethane removed via rotary evaporation (22 ℃, 20 mmHg) (Note 11) until a free-flowing powder remains (Figure 3B).
Figure 3. (A) Mixture of linalool, linalyl acetate, and farnesol; (B) Mixture of compounds deposited on Celite (photos provided by the authors)
Equilibration of the column. A 19:1 mixture of hexanes (Note 12) and ethyl acetate (Note 13) is prepared in a graduated cylinder, and 300 mL is added to the column via a powder funnel. The eluent is poured down the walls of the column so as to minimally disturb the layer of silica gel. Air pressure is applied to the column via a 24/40 ground glass inlet adapter, forcing the solvent through the dry silica gel (Figure 4A). A rapid flow rate can be achieved by loosely applying the inlet to the top of the column. Under no circumstances should the inlet be tightly set in the ground glass joint as an explosion can result. The eluent forced through the column is collected in a 500-mL Erlenmeyer flask. When the top of the solvent layer reaches the top of the silica gel layer, the stopcock is closed, the inlet adapter removed, and the contents of the Erlenmeyer flask poured back into the column. This process is repeated (typically three times) until a 1-cm high layer of solvent remains above the top of the silica gel and none of the silica gel in the column is dry (Figure 4C).
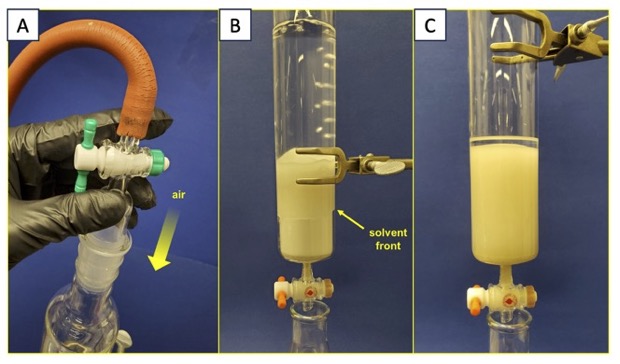
Figure 4. Equilibration of the silica gel column. (A) Application of air pressure; (B) Solvent front moving down the column; (C) Column after equilibration (photos provided by the authors)
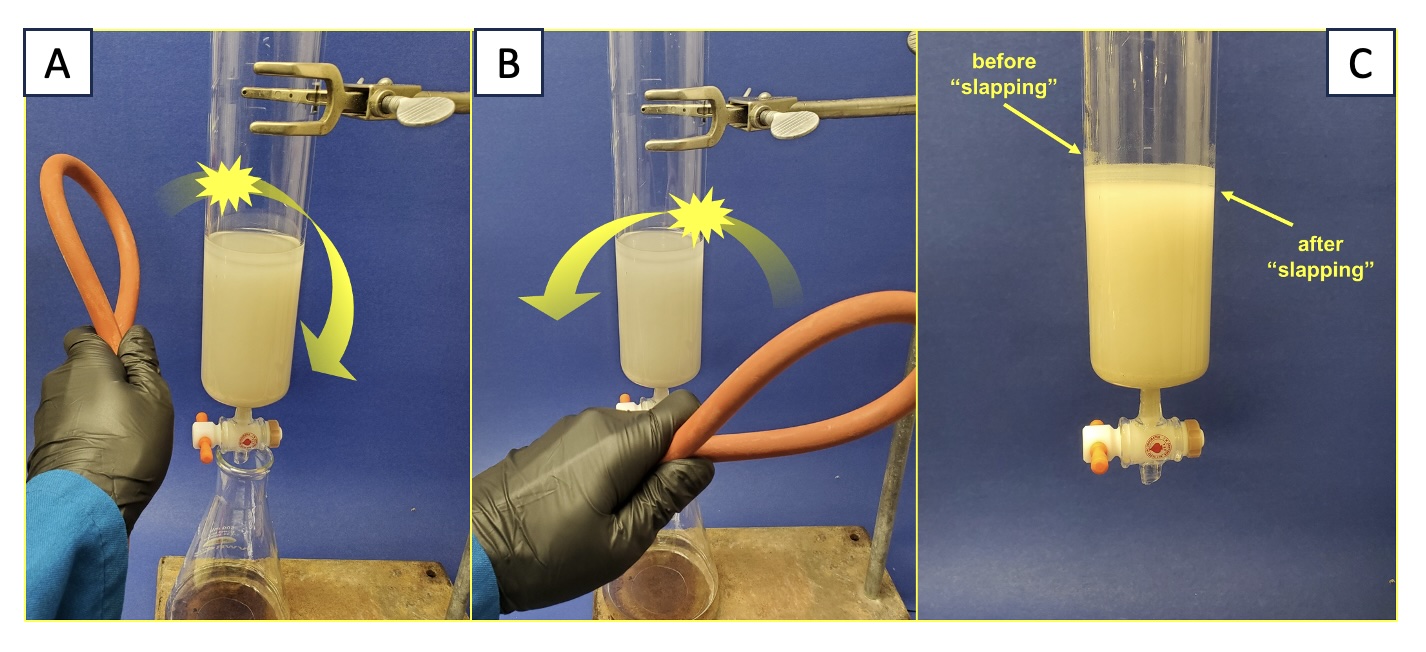
With the stopcock closed, the column is next "slapped" with a folded length of red rubber tubing to promote the removal of air bubbles and to improve the packing of silica gel particles (
Video 1). The column is repeatedly struck with a forehand-backhand motion that results in the silica gel column becoming more compact (Figure 5). Between each cycle of slapping, the top of the solvent layer is pushed down using air pressure until it returns to just reaching the top of the silica gel. This process is complete when additional slapping no longer causes the height of the silica gel column to contract, and the top of the solvent layer remains level with the top of the silica gel. It is important
not to allow the level of solvent to drop below the level of the top of the silica gel and the top of the silica gel should be even and flat.
Dry-loading the sample. Sand (20 g) is very slowly added to the top of the column, being careful not to disturb the level surface of silica gel (Note 14). Eluent (19:1 hexanes/ethyl acetate) (5 mL) is introduced by disposable Pasteur pipette down the walls of the column to wash down any loose grains of sand. The resulting layer of sand is ca. 15 mm in height. Using air pressure, the top of the solvent layer is forced into the sand layer until the top of the sand layer just begins to appear visibly dry (Figure 6B).
The mixture of linalool, linalyl acetate, and farnesol deposited on Celite is added to the top of the sand layer via a powder funnel. A metal spatula is used to scrape off Celite that adheres to the sides of the recovery flask, and the flask is rinsed with two 5-mL portions of eluent (the washes are added down the wall of the column via a Pasteur pipette). The Celite layer is made level by "slapping" the column with red rubber tubing, and the top of the solvent layer is pushed down with air pressure until it just reaches the top of the Celite layer. Additional sand (20 g) is carefully added to create a layer ca. 15 mm in height over the top of the Celite layer. Once again, 5 mL of eluent is added down the side of the column via a Pasteur pipette to wash any loose grains of sand down onto the upper layer of sand (Figure 6C).
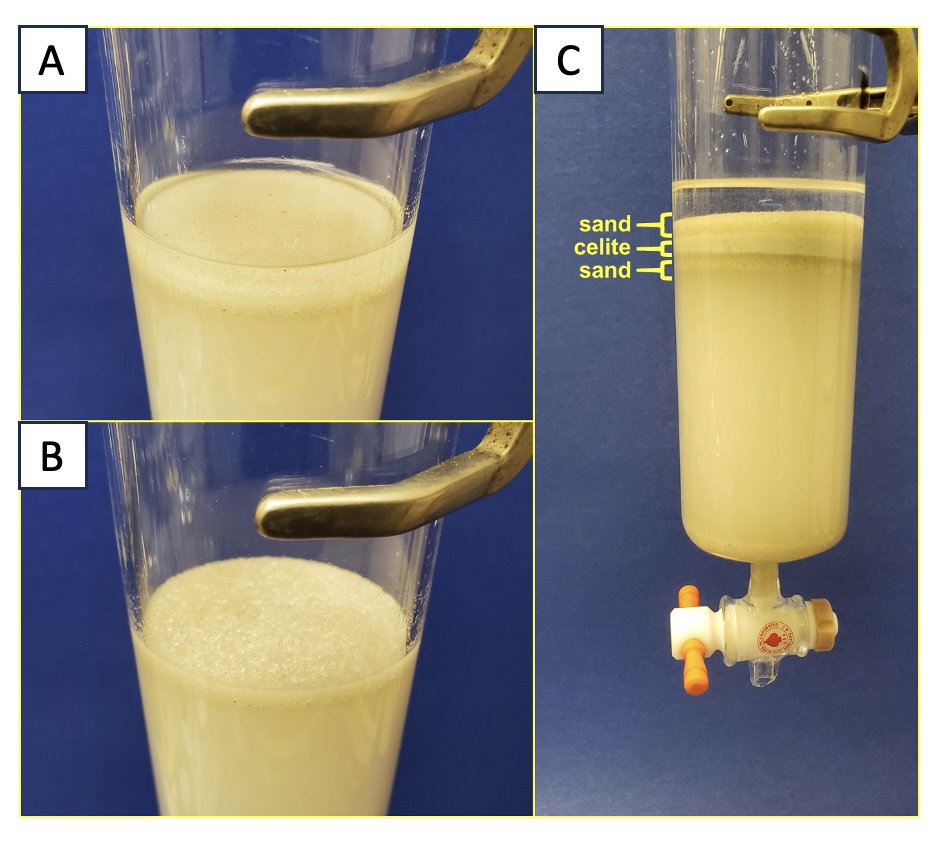
Figure 6. Loading the mixture of compounds. (A) Column prior to addition of compounds on Celite; (B) Solvent lowered to level of the sand; (C) Compound loaded and ready for elution (photos provided by the authors)
Elution and collection of fractions. Eluent (ca. 500 mL) is carefully added to the column without disturbing the top layer of sand. The Erlenmeyer collection flask below the column is replaced with a test tube (
Note 15), and the position of the column is adjusted so that the test tube is able to stand upright on the work surface with the mouth of the test tube resting on the outlet of the column (Figure 7). The stopcock is opened, air pressure is applied to column, and 40-mL fractions are collected at a flow rate of ca. 5 cm of solvent level decrease per min (20 seconds per fraction) (
Video 2).
Figure 7. Collection of a fraction (photo provided by the authors). Video 2.
Rate of fraction collection (video provided by the authors)
Fraction collection is continued for a total of 60 fractions which are subjected to thin layer chromatographic (TLC) analysis (Figure 8). The less polar impurity, linalyl acetate, elutes in early fractions, followed by linalool, and finally farnesol, the more polar impurity. Fractions containing linalool are combined in a 2-L, one necked, round-bottomed flask. Each test tube containing compound is rinsed with ca. 5 mL of dichloromethane which is added to the collection flask. The combined fractions are concentrated via rotary evaporation (30 ℃, 75 mm Hg), and the resulting oil is transferred via Pasteur pipette to a tared, oven-dried 20-mL storage vial with the aid of dichloromethane. The solvent is removed via rotary evaporation (30 ℃, 75 mm Hg) to afford 0.95 g of linalool as a clear colorless oil (95% recovery, not corrected for the 97% purity of linalool used) (Figure 9) (Notes 16, 17, 18, 19).
Figure 8. TLC analysis of column fractions (photo provided by the authors)
Figure 9. Purified linalool (photo provided by the authors)
B. Purification of linalool by column chromatography with gradient elution.
Wet-packing and equilibrating the column. A 40-mm diameter glass column (60 cm in length) with a Teflon stopcock and sintered glass frit is secured by two clamps such that the column is perpendicular to the work surface and the spout is slightly above a test tube (Note 20) rack placed underneath the column (Figure 10A). Because a gradient elution is planned, mobile phases of increasing polarity are prepared prior to beginning the column. Because optimal conditions for isocratic elution were determined to be 5% ethyl acetate/95% hexanes (Notes 21, 22), the gradient column began with 2.5% ethyl acetate/97.5% hexanes, or half of the polarity for isocratic elution - this should result in superior separation between the top spot (linalyl acetate, "impurity") and middle spot (linalool, target). A total of 500 mL of this initial eluent mixture is prepared, and this is used to pack the silica and load the crude mixture (see below) as well as elute the first fractions. A total of 1000 mL of 5% ethyl acetate/95% hexanes is also prepared in addition to 650 mL of 15% ethyl acetate/85% hexanes, which should facilitate quick elution of the bottom spot (farnesol, "impurity") after the target spot has finished eluting (Figure 10B).

Figure 10. (A) Glass chromatography column positioned above test tube rack; (B) Mobile phases of increasing polarity prepared prior to running the column (photo provided by the authors)
The dry silica gel (84 g) (Note 23) is weighed into a separate 500-mL Erlenmeyer flask, and a slurry is formed by the addition of the lowest polarity eluent (250 mL). This slurry is mixed by swirling the flask until no air bubbles are observed (Figure 11A), and the slurry is quickly poured into the column. The column is drained by opening the stopcock and applying gentle air pressure via a rubber stopper with a hole connected to an air hose (Figure 11B).
Figure 11. (A) Slurry of silica gel in first mobile phase; (B) Rubber stopper with hole and adapter for air hose (photos provided by the authors)
The eluent that is forced through the column is collected in the same 500-mL Erlenmeyer flask used to prepare the slurry, and the residual silica gel is made into a second, smaller slurry that is re-applied to the column. The solvent is again drained until a 1-cm high layer of solvent remains above the top of the silica gel, and the "slapping" procedure described in Part A is performed to ensure a level layer of silica gel. After the silica is leveled and the solvent is drained until it is level with the silica gel, a layer of 2 cm of Na2SO4 (Note 24) is applied to protect the baseline from disturbances which may be caused by addition of the mobile phase (Figure 12). Finally, 5 mL of the low polarity mobile phase is applied to the Na2SO4 layer, and the "slapping" procedure is repeated to ensure a level layer of Na2SO4.
Figure 12. Addition of the silica slurry to the column, followed by solvent elution and addition of Na2SO4 to protect the baseline (photos provided by the authors)
Wet-loading the sample. A 50-mL, one-neck, pear-shaped flask is charged with linalool (1.00 g, 6.48 mmol) (Note 6), linalyl acetate (0.10 g, 0.51 mmol) (Note 7), and farnesol (0.10 g, 0.45 mmol) (Note 24). A 5-mL portion of the least polar eluent is used to dissolve the mixture (Figure 13A), and this solution is applied via disposable Pasteur pipette directly to the Na2SO4 layer of the packed column (gentle dripping and gently rinsing down the walls of the column ensures the silica baseline is not disturbed). Gentle air pressure is applied until the solvent level is again even with the Na2SO4 at the top of the column. This is repeated twice more by rinsing the flask with 5-mL portions of the least polar component, which ensures the crude mixture is adsorbed to the silica and not simply dissolved in the minimal eluent remaining above the silica.

Figure 13. (A) Mixture of linalool, linalyl acetate, and farnesol dissolved in mobile phase; (B) Collection of fractions by sliding test tube rack below the column (photos provided by the authors)
Elution and collection of fractions. The least polar eluent (ca. 250 mL of the originally prepared 500 mL remaining after the packing and loading process) is added in portions to the remaining space in the column above the silica (about 100 mL can fit in this space for this particular column). The Erlenmeyer flask used during the packing and loading process is replaced with a test tube rack positioned below the column. The stopcock is opened, air pressure is applied to the column, and 50-mL fractions are collected at a linear column flow rate of ca. 5 cm of solvent decrease per minute. The test tube rack is simply slid underneath the spout of the column with no arresting of the flow between fractions (Figure 13B).
After the remainder of the low polarity eluent is depleted (until fraction 5), the eluent of medium polarity (5% ethyl acetate/95% hexanes here, the optimum system for isocratic elution) is loaded as described above, and fractions are eluted until the target spot has completely finished eluting. In this column, this corresponded to ca. 1000 mL. At this point, the most polar eluent mixture is used until the bottom spot is finished eluting, which corresponds to an additional ca. 650 mL of eluent for this column.
The less polar impurity, linalyl acetate, eluted in early fractions (8-9) and the more polar impurity, farnesol, eluted in later fractions (31-33). Fractions containing linalool (13-22) are combined in a 1-L, one necked, round-bottomed flask. The combined fractions are concentrated via rotary evaporation (30 ℃, 75 mm Hg), and the resulting oil is transferred to a tared 25-mL recovery flask with the aid of diethyl ether. Concentration by rotary evaporation (30 ℃, 75 mm Hg) affords 0.97 g of linalool as a clear colorless oil (97% recovery, not corrected for the 97% purity of linalool used for the column) (Notes 26, 27).
Figure 14. TLC plates of all collected fractions, stained with Hanessian's stain (photo provided by the checkers)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/about/governance/committees/chemical-safety.html. In the case of this procedure, risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
linalool,
linalool acetate,
farnesol, silica gel,
ethyl acetate, hexanes,
toluene, and
dichloromethane.
2. Washed sand was purchased by the authors from Fisher Chemicals and was used as received.
3. The authors purchased SiliaFlash P60 (40-63 μm, 400-230 mesh) silica gel from Silicycle and used it as received. The checkers purchased silica gel (40-63 μm, 400-230 mesh, technical grade) from Sigma-Aldrich and used it as received.
4.
Linalool (97%, racemic) was purchased by the authors from Sigma-Aldrich and was used as received.
5.
Linalyl acetate (>97%, racemic) was purchased by the authors from Sigma-Aldrich and was used as received.
6.
Farnesol (95%) was purchased by the authors from Sigma-Aldrich and was used as received.
7.
Dichloromethane (>98.5%) was purchased by the authors from Sigma-Aldrich and was used as received.
8. TLC analysis was performed with silica gel plates (glass backed, 250 μm) purchased from Sorbtech with hexanes-
ethyl acetate as the eluent and visualization with PMA stain (prepared by dissolving 10 g of
phosphomolybdic acid in 100 mL of ethanol) (Figure 15).
Figure 15. TLC analysis (visualization with PMA) of mixture of linalyl acetate (top spot), linalool, and farnesol (bottom spot, mixture of stereoisomers) using (A) 19:1, (B) 12:1, (C) 9:1, and (D) 4:1 hexanes/ethyl acetate as eluent (photo provided by the authors)
9. Visualization of TLC plates can be performed with (A)
KMnO4, (B) Hanessian stain, (C)
Iodine on silica gel, and (D) PMA. The TLC stains were prepared as follows: (A) 1.5 g
KMnO4, 10 g
K2CO3, 1.25 mL 10%w/v
NaOH, in 200 mL
H2O; (B) 12 g
ammonium molybdate, 0.5 g ceric
ammonium molybdate, 15 mL conc
H2SO4 in 235 mL
H2O; (D) 10 g of
phosphomolybdic acid in 100 mL of
EtOH.
Figure 16. TLC analysis of linalyl acetate ("LA"), linalool ("L"), farnesol ("F"), and mixture ("MX") using (A) KMnO4 solution; (B) Hanessian's stain; (C) Iodine on silica gel; and (D) PMA (elution with 9:1 hexanes/ethyl acetate) (photo provided by the authors)
10. Celite was purchased by the authors from EMD Chemicals and was used as received.
11. The rotary evaporator should be equipped with a bump guard since bumping of the mixture is sometimes observed during concentration of the sample onto Celite. If this is a persistent problem, increasing the temperature to 30 ℃ may reduce bumping.
12. Hexanes (>98.5%, mixture of isomers) was purchased by the authors from Sigma-Aldrich and was used as received. Checkers purchased hexanes from Fisher Chemical (ACS Grade) and used as received.
13.
Ethyl acetate (>99.7%) was purchased by the authors from Sigma-Aldrich and was used as received.
14. To avoid disturbing the top of the silica gel it may be helpful to have several cm of solvent above the level of the silica gel while the sand is added.
15. Test tubes (25 x 150 mm, ca. 60 mL capacity) were purchased by the authors from VWR.
16. The authors found that it was necessary to azeotrope the recovered
linalool using
toluene directly after the column in order to remove water that was introduced to the sample during the column.
Toluene (99.5% purity) was purchased from J. T. Baker and was used as received. Without the azeotroping procedure, the purity of recovered linalool was determined to be 95% by qNMR analysis, and a water peak was visible on the NMR spectrum. The checkers did not find this to be necessary.
17.
Linalool has a boiling point of 198-199 ℃. The checkers found that mass recovery was lower when the final sample was left at reduced pressure for extended time (e.g., at 10 mmHg for 11 h).
18.
Linalool has the following physical and spectroscopic properties: R
f = 0.17 in 19:1 hexanes-
ethyl acetate and 0.3 in 9:1 hexanes-
ethyl acetate (
Note 8);
1H NMR
pdf (CDCl
3, 400 MHz) δ: 5.91 (dd, J = 17.3, 10.7 Hz, 1H), 5.21 (dd, J = 17.3, 1.3 Hz, 1H), 5.12 (apparent ddquin, J = 7.2, 5.8, 1.4 Hz, 1H), 5.06 (dd, J = 10.8, 1.3 Hz, 1H), 2.20 - 1.84 (m, 2H), 1.68 (d, J = 1.3 Hz, 2H), 1.65 - 1.53 (m, 3H), 1.59 - 1.46 (m, 2H), 1.28 (s, 3H);
13C NMR
pdf (CDCl
3, 100 MHz) δ: 145.2, 132.1, 124.5, 111.8, 73.6, 42.2, 28.0, 25.9, 22.9, 17.8; FTIR (neat) (cm
-1): 3385, 2969, 2918, 2858, 1451, 1411, 1374, 1112, 995, 918, 835, 688.
pdf19. The purity of the recovered
linalool was determined to be 99% by qNMR
pdf analysis using a sample prepared by dissolving 43.4 mg of the product and 31.1 mg of
dimethyl terephthalate (>99%) in 1.0 mL of CDCl
3 in an oven-dried glass vial.
20. Test tubes (25 x 150 mm, ca. 60 mL capacity) were purchased by the authors from VWR.
21.
Ethyl acetate (>99.5%) was purchased by the authors from Fisher Scientific and was used as received.
22. Hexanes (>98.5%, mixture of isomers) was purchased by the authors from Fisher Scientific and was used as received.
23. The authors purchased SiliaFlash F60 (40-63 μm, 230-400 mesh) silica gel from Silicycle and used it as received. The checkers purchased silica gel (40-63 μm, 400-230 mesh, technical grade) from Sigma-Aldrich and used it as received.
24.
Na2SO4 was purchased from Sigma-Aldrich and used as received.
25.
Farnesol (95%,
trans-trans stereoisomer) was purchased by the authors from Sigma-Aldrich and was used as received.
26.
Linalool has the following physical and spectroscopic properties: R
f = 0.17 in 19:1 hexanes-
ethyl acetate and 0.3 in 9:1 hexanes-
ethyl acetate;
1H NMR (CDCl
3, 400 MHz) δ: 5.91 (dd, J = 17.3, 10.7 Hz, 1H), 5.21 (dd, J = 17.3, 1.3 Hz, 1H), 5.12 (apparent ddquin, J = 7.2, 5.8, 1.4 Hz, 1H), 5.06 (dd, J = 10.8, 1.3 Hz, 1H), 2.20 - 1.84 (m, 2H), 1.68 (d, J = 1.3 Hz, 2H), 1.65 - 1.53 (m, 3H), 1.59 - 1.46 (m, 2H), 1.28 (s, 3H).
27. The purity of the recovered
linalool was determined to be 97% by qNMR analysis using a sample prepared by dissolving 15.7 mg of the product and 18.2 mg of
1,3,5-trimethoxybenzene (>99%) in 1.0 mL of CDCl
3.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Column chromatography reigns supreme in the modern research laboratory as the most widely employed method for the purification of organic compounds on a preparative scale. Approximately 60% of the articles published in
Organic Syntheses over the past two years deploy "manual" silica gel columns for the purification of reaction products. "Flash column chromatography", a variant popularized by Still and coworkers in 1978,
3 continues to be the most common method for chromatographic purification in spite of the increasing use of automated systems with pre-packaged columns.
This inaugural OS Techniques article provides two detailed and reliable protocols for carrying out flash column chromatography. A test sample of 1 g of the sesquiterpene linalool is prepared containing 100 mg each of farnesol and linalyl acetate as contaminants and then subjected to purification by flash column chromatography. Linalool is recovered in over 95-97% yield with 97-99% purity by using either isocratic or gradient elution. As is the case with all results published in Organic Syntheses, the purity and yield (recovery) of linalool has been independently "checked" for reproducibility and verified in the laboratory of a member of the Organic Syntheses Board of Editors.
The separation of linalool from farnesol is relatively challenging (ΔRf = 0.09 in 19:1 hexanes-ethyl acetate) and was selected as representative of typical separations encountered in preparative synthetic organic chemistry research. The choice of these common, commercially available compounds and the publication of verified results provides the reader with an opportunity to practice and test their skill at this essential technique in synthetic organic chemistry.
Several key decisions must be made by researchers as they formulate plans for the purification of a compound by flash column chromatography. The discussion below provides general guidance for carrying out a purification by flash chromatography and is organized in the order in which these key questions are generally considered. Photos illustrating many aspects of this discussion can be found in the experimental procedures presented above. More detailed discussion and further guidance is available in Still et al.'s original paper
3 and in a number of excellent resources on flash chromatography that can be found online and in print.
4(1) Selection of the Eluent and Isocratic vs. Gradient Elution
The first decision the researcher must make in planning a column is whether to employ isocratic or gradient elution. Isocratic elution, in which the composition of the eluent remains constant throughout the chromatography, is operationally more convenient and often provides good resolution of the desired compound from impurities. Gradient elution, in which the polarity of the eluent is increased in a linear or stepwise fashion, is particularly useful when the mixture being subjected to chromatography includes multiple components with relatively different R
f values. In this case band broadening can be minimized and better resolution obtained by employing a solvent gradient of gradually increasing polarity for elution. Note that gradient elution often permits the use of lower amounts of silica gel as compared to isocratic elution although larger total volumes of eluent are generally required.
6 Typical techniques for performing flash chromatography with each mode of elution are illustrated in the two alternative procedures described in this article.
The choice of eluent is one of the most important decisions a researcher must make and TLC analysis of the crude mixture to be purified is an essential step in planning a flash column. In their original paper, Still et al. recommended choosing a solvent system for isocratic elution in which the key compound has a TLC Rf of 0.35, or 0.25 in the case of more challenging separations. In general, we recommend the choice of an eluent that provides an Rf of 0.2 to 0.3 for the key compound of interest. More polar eluents tend to run the key component through the column too quickly and result in diminished resolution. On the other hand, less polar eluents (Rf < 0.2) allow the compound to reside too long in the column and can result in band broadening due to diffusion and reduced resolution. In the procedure in Part A of this article, we chose 19:1 hexanes-ethyl acetate as the isocratic eluent, a solvent system in which linalool displays an Rf value of 0.2.
Silica gel particles display silanol groups on their surface and exhibit weak acidity. Although we do not employ this stratagem in this article, in some cases it is useful to "spike" the eluent (and/or the solvent used to equilibrate the silica gel column) with small amounts (0.5 to 5%) of triethylamine. This is particularly valuable in the case of the purification of acid-sensitive compounds and with compounds that incorporate basic functionality and exhibit "tailing" of spots on TLC analysis. In a similar fashion, the addition of 0.5 to 5% formic or acetic acid can reduce tailing and improve resolution in the chromatographic purification of compounds whose structures include acidic functional groups.
(2) Choice of Amount and Type of Silica Gel
An essential feature of flash column chromatography is the use of silica gel with particle size 40 to 63 μm (230-400 mesh) rather than the coarser grade of silica gel (70-230 mesh, 63-200 μm) that had been in common use prior to the publication by Still and coworkers. This finer grade of silica gel has a higher surface area per weight and affords more efficient separation than coarser grades of silica gel. Interestingly, Still et al. found that silica gel with particle sizes less than 40 μm offers no improvement in resolution.
A number of different suppliers offer silica gel suitable for flash chromatography. Part A of this article employs SiliaFlash P60
5 from SiliCycle while Part B uses the F60 grade from the same supplier. The F60 grade has lower metal content and a tighter particle size distribution affording somewhat better resolving power. However, F60 silica gel is twice the price of P60 material and for most purposes P60 silica gel provides satisfactory results. The Checkers found that technical grade silica gel (40 to 63 μm) was equally effective.
The number of components to be separated determines the ratio of silica gel to crude sample mixture that is required. Those components of a mixture that have a large difference in Rf from the key analyte of interest can often be ignored when choosing the amount of silica gel for the column. Specifically, the ratio of silica gel is chosen without concern for components that effectively stay at the baseline or elute near the solvent front on TLC analysis using the eluent. A 20:1 ratio of silica gel to compound mixture often suffices for easy separations, while ratios of up to 100:1 or 120:1 may be required to separate compounds with nearly overlapping spots on TLC analysis. For the purification of linalool in this article (ΔRf = 0.09 from farnesol in 19:1 hexanes-ethyl acetate) a ratio of 70:1 silica gel to the crude mixture was found to be optimal.
(3) Column Dimensions
Once the researcher decides the amount of silica gel to be used the next step is to choose a column of appropriate diameter. Longer columns with smaller diameters result in the compound mixture being applied in a band of greater length with potential loss of resolution. In addition, "long and thin" columns typically produce more back-pressure during elution, since solvent has to flow through a longer length of stationary phase. This back-pressure can then reduce the flow rate with a detrimental effect on the resolution of bands due to diffusion. Wider diameter columns in principle allow the crude mixture to be applied in a thinner layer, but in practice achieving a uniform thin initial band can prove challenging. In practice, many researchers aim for a column with "aspect ratio" (height to width) of between 1.5:1 and 3:1.
(4) Method for Packing the Column
Some chromatography columns come equipped with a glass frit at the bottom as illustrated in the column used in Part B of this article. When using columns with glass frits it is important to choose a column that does not have significant dead volume between the bottom of the frit and the stopcock. In the case of columns without a frit, the bottom of the column must be blocked with a plug of glass wool or dry cotton. The plug is then covered with a minimum amount of commercially available acid-washed sea sand to achieve an even flat surface at the bottom of the column.
Two general procedures are available for packing the column with silica gel: "wet-packing" and "dry-packing". In both cases it is essential that the column of silica gel and eluent be prepared free of any bubbles and channels prior to the loading of the sample to be purified.
"Wet-packing", by far the most common method employed in recent procedures published in Organic Syntheses, is illustrated in Part B of this article. In this case a well-mixed slurry of the silica gel is prepared in a beaker or Erlenmeyer flask and then poured into the column. Thorough mixing is required to remove air bubbles and to ensure equilibration of the silica gel with the eluent.
"Dry-packing" is usually simpler operationally as compared to wet-packing and often gives equivalent results. As illustrated in Part A, in this case dry silica gel followed by solvent is added to the column which is then pressurized and finally "slapped" with rubber tubing as described in detail in the text and video. This agitation of the column, which is also required when employing wet-packing, is essential to ensure the removal of air bubbles and complete equilibration of the silica gel and initial eluent. Failure to complete this step can result in significant reduction in the resolving power of the column.
Finally, the top of the silica gel column is capped with a very thin layer of either sand (Part A) or sodium sulfate (see Part B) to protect the surface of the silica gel when additional solvent is added. Though more expensive, sodium sulfate forms a firmer protective crust at the top of the silica gel column that some researchers find beneficial.
(5) Method for Loading the Compound
Three methods are commonly employed to apply the sample to be purified to the column; in this discussion we refer to these methods as "neat-loading", "wet-loading", and "dry-loading".
"Neat-loading" involves careful addition of a liquid sample (without solvent) to the top of the column, typically via disposable pipette. This approach obviously is only applicable to free-flowing liquids and is best reserved for non-polar compounds since the exothermicity of adsorption of neat polar compounds on silica gel at the top of a column may cause channels and cracks to develop that reduce resolution.
Adding the sample as a solution in a small amount of solvent ("wet-loading") is a more common procedure and is illustrated in detail in Part B of this article. Ideally the initial eluent is employed as the solvent and the use of a minimum quantity of solution ensures that the sample is loaded as a band of minimal thickness. Typically, a 20-25% solution of the crude sample mixture is employed.
"Dry-loading" provides an attractive vehicle for applying samples when the crude sample mixture has limited solubility in the initial eluent. Dry-loading also facilitates the application of samples as particularly thin layers at the top of the column. A solution of the sample, often in dichloromethane, is deposited onto a solid support by rotary evaporation to obtain a free-flowing solid. The solid is then carefully added to the top of the chromatography column as detailed in the example in Part A of this article. Silica gel is the most common adsorbent used for this purpose, but as illustrated in Part A, the use of Celite provides a non-acidic alternative that may be preferable in the case of compounds sensitive to acid. Celite also has the advantage that it does not form significant interactions with organic molecules and may allow for the deposition of the sample as an extremely thin layer on the silica gel once elution begins.
(6) Flow Rate for Elution and Size of Fractions
The crux of "flash chromatography" is that the manual purification of organic compounds can be rapidly and conveniently achieved by using columns of 40 to 63 μm silica gel and employing a rapid flow rate by application of compressed air rather than by relying on gravity as in conventional column chromatography. In their original paper, Still et al. recommended a flow rate such that the level of solvent above the silica gel column drops at a rate of ca. 2 inches per minute.
The optimal flow rate for a chromatographic separation is determined by a balance in which the broadening of bands by diffusion is minimized while allowing sufficient time for full equilibration between the mobile and stationary phases.
7 For the procedures in this article we chose a flow rate of ca. 5 cm per minute, achieved by application of compressed air. Care must be taken to ensure that sources of "house" compressed air are free of oil and other contaminants, and for this reason some researchers prefer to use a pressurized inert gas (e.g., nitrogen) rather than air for flash columns. Note also that the gas inlet must not be tightly secured to the column without a pressure relief valve or an explosion can result.
A general rule of thumb is to collect fractions in which the volume per fraction in mL is set equal to one-half the amount of silica gel in grams. For the purification of linalool in this article, fractions of 40 to 50 mL were collected with a column containing 84 g of silica gel. Contrary to common belief, collecting smaller size fractions will not lead to improved separation of components. Finally, an important point that must be stressed is that once a sample is applied to the column and elution is begun, elution should not be suspended (for more than a very brief time) because diffusion will broaden bands and detrimentally impact resolution.
Fractions containing the desired compound(s) are generally identified by TLC analysis. Combination of the appropriate fractions and concentration then furnishes the purified compounds. Researchers should be alert for the presence of small amounts of water in the purified products from some silica gel columns. Whether this is a problem appears to vary with different compounds, different batches of silica gel, and the polarity and composition of the eluent. In Part A of this article the authors noted that the chromatographed linalool could not be obtained in 99% purity unless the combined fractions from the column were azeotroped with toluene several times to remove traces of water; however, the checkers did not have this problem. The presence of water also proved to be a problem in the purification of a compound in prior work of one of the authors where traces of water acquired during flash chromatography interfered with the next step in a synthetic sequence.
8
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Linalool; (78-70-6)
Linalyl acetate; (115-95-7)
Farnesol; (4602-84-0)

|
Benjamin D. Senzer received an A.B. degree in Chemistry at Harvard College in 2020. He is currently pursuing a Ph.D. degree in the Department of Chemistry at the Massachusetts Institute of Technology under the direction of Professor Rick Danheiser. The main focus of Ben's current research is the total synthesis of natural products using strained and unusual molecules. |

|
Cholapat Varongchayakul was born in Chonburi, Thailand in 2001. As an undergraduate at the Massachusetts Institute of Technology he joined the laboratory of Professor Rick Danheiser in 2020 and received his B.S. degree in chemistry in 2024. |

|
Brian Daniels received a B.S. degree in Chemistry from The Ohio State University in 2020. He is currently pursuing a Ph.D. degree in the Department of Chemistry at the University of California, Irvine under the direction of professor Vy Dong. The main focus of Brian's current research is the development of catalytic, asymmetric methods related to phosphorus stereocenters. |

|
Rick L. Danheiser received his undergraduate education at Columbia where he carried out research in the laboratory of Professor Gilbert Stork. He received his Ph.D. at Harvard in 1978 working under the direction of E. J. Corey on the total synthesis of gibberellic acid. Dr. Danheiser is the A. C. Cope Professor of Chemistry at MIT where his research focuses on the design and invention of new annulation and cycloaddition reactions, and their application in the total synthesis of biologically active compounds. |

|
Vy M. Dong studied organic chemistry with Larry Overman at UC Irvine (B.Sc. 1998). She obtained her doctorate degree with David MacMillan at Caltech and then went on to postdoctoral studies with Robert Bergman and Kenneth Raymond at UC Berkeley. Vy began her independent career at the University of Toronto and returned to UC Irvine as a full professor, where her lab focuses on catalysis She's currently an editor with Organic Syntheses and Chemical Society Reviews. |

|
Katerina Scotchburn received a B.Sc. in Biopharmaceutical Science from the University of Ottawa in 2021. She is currently pursuing a Ph.D. with Professor Sophie Rousseaux at the University of Toronto. Her research interests include base-metal catalysis and the synthesis of small rings. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved