Org. Synth. 2003, 80, 104
DOI: 10.15227/orgsyn.080.0104
RUTHENIUM-CATALYZED ALKYLATION OF AROMATIC KETONES WITH OLEFINS: 8-[2-(TRIETHOXYSILYL)ETHYL]-1-TETRALONE
[(1(2H)-Naphthalenone, 3,4-dihydro-8-[2-(triethoxysilyl)ethyl]-)]
Submitted by Fumitoshi Kakiuchi and Shinji Murai
1.
Checked by Dennis P. Curran and Andre Lapierre.
1. Procedure
8-[2-(Triethoxysilyl)ethyl]-1-tetralone (Note 1). An apparatus, consisting of a 100-mL, two-necked, round-bottomed flask, reflux condenser connected to a vacuum/N2 line, inlet tube sealed with a rubber septum, and magnetic stirring bar, is evacuated, then flushed with nitrogen. This cycle is repeated four times. The apparatus is flame-dried under a flow of nitrogen, then cooled to room temperature under a nitrogen atmosphere. Carbonyldihydridotris(triphenylphosphine)ruthenium(II), RuH2(CO)(PPh3)3, (0.918 g, 1.00 mmol, 0.010 eq) (Notes 2, 3) is placed in the flask under a slow flow of nitrogen. Addition of 20 mL of toluene (Note 4) to the flask affords a suspension of white solids (Note 5). To this suspension are added triethoxyvinylsilane (20.93 g, 110 mmol, 1.10 eq) (Notes 6, 7), then 1-tetralone (14.62 g, 100 mmol, 1.00 eq) (Notes 6, 8) at room temperature through the rubber septum using a syringe. The resulting mixture containing the white solids is heated to reflux in an oil bath (oil bath temperature: 135°C) (Notes 9, 10). The reaction mixture becomes colorless within 1 min, then changes to a dark wine red color within 5 min (Note 11). After heating for 30 min, the reaction mixture is cooled to room temperature. About half of the reaction mixture is transferred to a 50-mL, round-bottomed flask (Note 12) for concentration and distillation. Volatile materials (toluene and triethoxyvinylsilane) are removed by rotary evaporation under reduced pressure (2 mm) at 40°C. After almost all volatile materials are removed, the other half of the reaction mixture is transferred to the flask. The reaction vessel is rinsed with two 5-mL portions of toluene, then the rinses are transferred to the distillation flask. The combined reaction mixture and rinses are concentrated. Solvent and vinylsilane are removed by rotary evaporation under reduced pressure (2 mm at 40°C) (Note 13). Distillation of the residue under reduced pressure gives 31-32.5 g (92-96%) of 8-[2-(triethoxysilyl)ethyl]-1-tetralone as a pale yellow liquid, bp 133-135°C/0.2 mm (Notes 14-18).
2. Notes
1.
This procedure is a modification of one published by our group.
2,32.
The ruthenium complex,
carbonyldihydridotris(triphenylphosphine)ruthenium(II), can be synthesized according to the literature method.3,4 This complex is commercially available from Aldrich Chemical Company, Inc. and Strem Chemicals, Inc.3.
The commercially available
ruthenium complex (Strem Chemicals, Inc.) and the complex prepared in the submitters' laboratory showed comparable catalytic activity. The checkers purchased the complex from Strem Chemicals, Inc.4.
Toluene was dried over CaH2, then distilled under a N2 atmosphere.
5.
The
ruthenium complex is only slightly soluble in
toluene at room temperature.
6.
Triethoxyvinylsilane, 1-tetralone, and toluene were purchased from commercial suppliers (Aldrich Chemical Company, Inc., Strem Chemicals, Inc., Chisso Co., or Wako Pure Chemical Industries, Ltd.).
7.
The silane was dried over CaH2, then distilled under reduced pressure (
bp = 70°C/6 mm).
8.
1-Tetralone was dried over CaSO4, then distilled under reduced pressure (
bp 85°C/2 mm).
9.
Maintaining the oil bath temperature above 130°C (preferentially around 135°C) is necessary to effect the catalytic reaction with good reproducibility.
10.
The actual temperature of the reaction mixture was around 125°C throughout the reaction.
11.
The color of the reaction mixture changes to dark green, wine red, and finally dark wine red within 5 min.
12.
The use of a small flask is recommended to minimize loss of the product.
13.
The checkers transferred the entire reaction mixture to a
100-mL, round-bottomed flask with the aid of 2 × 5-mL washes with
toluene. A
distillation apparatus with a three-way adapter and three collection vessels was attached, and the solvent was removed by gentle heating at 3 mm. The residue was then distilled as described.
14.
The second fraction is collected. The first fraction (
0.20-0.28 g;
65-100°C/0.2 mm) containing unknown impurities and a small amount of the desired product is discarded.
15.
The residue is prone to bump during the late stages of the distillation. Wine red splashes on the wall of the distillation head results in contamination of the product with impurities, including
triphenylphosphine,
triphenylphosphine oxide, and the ruthenium complex. If the product is contaminated with these impurities, it can be redistilled.
16.
Heating the distillation head with a heat gun is recommended to avoid reducing the yield of the product.
17.
The spectral properties are as follows:
1H NMR (300 MHz, CDCl
3) δ: 0.94-1.00 (m, 2 H, SiCH
2), 1.26 (t, 9 H, J = 6.97, CH
3), 2.05 (quintet, 2 H, J = 6.00, CH
2CH
2CH
2), 2.61 (t, 2 H, J = 6.33, ArCH
2), 2.90 (t, 2 H, J = 5.66, C(O)CH
2), 3.08-3.14 (m, 2 H, ArCH
2CH
2Si), 3.87 (q, 6 H, J = 5.58, OCH
2), 7.05 (d, 1 H, J = 7.17, ArH), 7.11 (d, 1 H, J = 7.47, ArH), 7.26-7.31 (dd, 1 H, J = 7.56, ArH);
13C NMR (75 Hz, CDCl
3) δ: 12.18 (SiCH
2), 18.21(CH
3), 22.81 (CH
2), 28.57, 30.82 [(SiCCH
2) and (ArCH
2)], 40.96 (C(O)CH
2), 58.18 (OCH
2), 126.73, 129.15, 130.31, 132.33, 145.68, 147.95 (Ar), 199.60 (C=O); MS (m/z): 336 (M
+), 290 (M
+ - EtOH), 189, 173, 163, 135, 115, 79, 63; IR (neat) cm
−1: 1680 (C=O). Anal. Calcd for C
18H
28O
4Si: C, 64.25; H, 8.39. Found: C, 64.24; H, 8.29.
18.
The checkers obtained
bp 160-168°C/2 mm.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure described here is typical for the catalytic alkylation of aromatic ketones at the ortho position by alkenes. Aromatic ketones are readily available by Friedel-Crafts acylation and many other methods,
5 and many of these ketones are suitable substrates for the present catalytic alkylation with alkenes affording the corresponding ortho-alkylated ketones.
3,6 The present method provides a direct way to alkylate aromatics with olefins. Moreover, the C-C bond formation takes place with exclusive ortho selectivity, while mixtures of o-, m-, p-isomers are usually obtained in the conventional Friedel-Crafts alkylation of aromatic compounds.
In the present
ruthenium-catalyzed reaction, a C-C bond is formed directly from a C-H bond without prior conversion of the C-H bond to another functional group such as halogen. The preparation of
8-[2-(triethoxysilyl)ethyl]-1-tetralone described above can be modified for a variety of substituted aromatic ketones. Some additional, representative examples of the RuH
2(CO)(PPh
3)
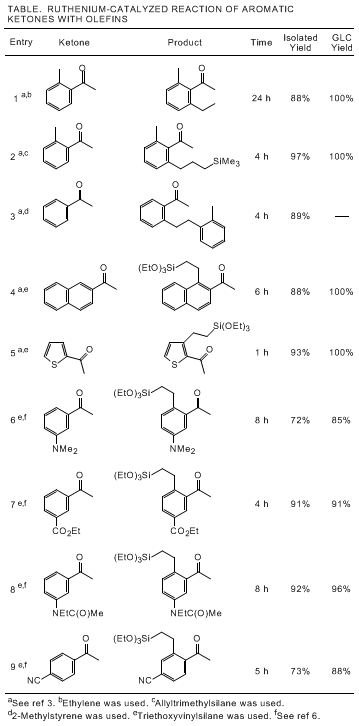
3-catalyzed addition of C-H bonds in aromatic ketones to olefins are shown in the Table.
3,6 Naphthalene derivatives and heteroaromatic ketones can be employed in the present coupling reaction. Functional group compatibility of this reaction is broad and both electron-donating and electron-withdrawing substituents are tolerated. In many cases, simple bulb-to-bulb distillation of the reaction mixture gives an analytically pure product. This simple one-pot procedure provides a new opportunity for site-selective alkylations of aromatic and heteroaromatic compounds.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
8-[2-(Triethoxysilyl)ethyl-1-tetralone:
1(2H)-Naphthalenone, 3,4-dihydro-8-[2-(triethoxysilyl)ethyl]- (9); (154735-94-1)
Carbonyldihydridotris(triphenylphosphine)ruthenium(II):
Ruthenium, carbonyldihydridotris(triphenylphosphine) (8,9); (25360-32-1)
Triethoxyvinylsilane:
Silane, ethenyltriethoxy- (9); (78-08-0)
1-Tetralone:
1(2H)-Naphthalenone, 3,4-dihydro- (8,9); (529-34-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved