Org. Synth. 2012, 89, 501-509
DOI: 10.15227/orgsyn.089.0501
Convenient Synthesis of α-Diazoacetates from α-Bromoacetates and N,N'-Ditosylhydrazine:
Preparation of Benzyl Diazoacetate
Submitted by Eiji Ideue, Tatsuya Toma, and Jun Shimokawa and
Tohru Fukuyama
1.
Checked by Pablo E.
Guzmán and Huw M.
L.
Davies.
.
1. Procedure
Caution! Diazoacetates are toxic and potentially explosive. They must be
handled with care. This preparation should be carried out in a
well-ventilated hood, and should be conducted behind a safety shield.
N,N'-Ditosylhydrazine (2).
A 3-L, three-necked round-bottomed flask equipped with an internal thermocouple temperature probe, a 20-mL pressure-equalizing dropping funnel sealed with a rubber septum, and an overhead mechanical stirrer fitted with a 8.5 × 2.0 cm rounded Teflon blade (Note 1) is charged under air with p-toluenesulfonyl hydrazide (1) (25.8 g, 134 mmol, 1.00 equiv) and p-toluenesulfonyl chloride (33.2 g, 174 mmol, 1.30 equiv) in 134 mL of CH2Cl2 (Note 2).
The suspension is stirred (165 rpm) in an ice bath while pyridine (14.1 mL, 13.8 g, 174 mmol, 1.30 equiv) is added dropwise from the dropping funnel over 5 min, such that the internal temperature does not exceed 10 °C.
During the addition, the reaction mixture becomes homogenous and turns yellow.
White precipitate is observed within 3 min and the reaction mixture is stirred for 30 min (Note 3).
n-Hexane (200 mL) and H2O (300 mL) are added and stirred in an ice bath for 15 min.
The white precipitates are collected in a medium-porosity sintered-glass Büchner funnel (diameter 90 mm) using suction filtration and washed with ice-cooled Et2O (200 mL), and then dried under vacuum (25 °C, 32 mmHg, 3 h).
The solid thus obtained is dissolved in boiling acetone (320 mL), and H2O (150 mL) is slowly added (Note 4).
The mixture is then cooled in an ice bath for 1 h and the white precipitates are collected by Büchner funnel (diameter 90 mm) with a medium-porosity fritted disk using suction filtration.
The precipitate is washed with ice-cooled Et2O (100 mL) and dried under vacuum over P2O5 (25 °C, 10 mmHg, 3 h) to give the first crop of N,N'-ditosylhydrazine (2) (35.5 g, 104 mmol, 78%).
A second crop of crystals (5.05 g, 14.8 mmol, 11%) is obtained by dissolving the concentrated mother liquor in hot acetone (60 mL) and H2O (27 mL) followed by the same cooling procedure (Note 5).
Benzyl bromoacetate (5).
A flame-dried, 1-L, three-necked round-bottomed flask is equipped with a 4.5-cm egg-shaped, Teflon-coated, magnetic stir bar, an internal thermocouple temperature probe, a 20-mL pressure-equalizing dropping funnel sealed with a rubber septum, and fitted with argon gas inlet.
The flask is flushed with argon and charged with sodium hydrogen carbonate (15.9 g, 189 mmol, 3.00 equiv), benzyl alcohol (6.50 mL, 6.80 g, 63.0 mmol, 1.00 equiv) and 310 mL of acetonitrile (Note 6).
The suspension is stirred in an ice bath while bromoacetyl bromide (7.70 mL, 88.2 mmol, 1.40 equiv) (Note 7) is added dropwise from a dropping funnel over 15 min.
The reaction mixture is stirred for 40 min and TLC analysis shows no starting material (Note 8).
Water (30 mL) is added and the reaction mixture is poured into CH2Cl2 (300 mL) and H2O (300 mL) in a 2-L separatory funnel.
The layers are separated, and the aqueous phase is extracted with an additional 300 mL of CH2Cl2.
The organic solutions are combined, washed with 300 mL of brine, and dried over sodium sulfate (80 g).
Filtration through a cotton plug, rinsing with 50 mL of CH2Cl2 and concentration on the rotary evaporator (35 °C, 50 mmHg, water bath) provides 14.6 g of the crude product as a clear amber oil.
This material is purified by silica gel column chromatography (elution with ethyl acetate/ n-hexane) (Note 9, 10).
The combined fractions are concentrated by rotary evaporation (40 °C, 27 mmHg) and then at 25 °C, 3.0 mmHg for 1 h to furnish 14.1 g (61.6 mmol, 98%) of benzyl bromoacetate (5) as a clear colorless oil.
(Note 11)
Benzyl diazoacetate (6).
A flame-dried, 1-L, three-necked round-bottomed flask is equipped with a 4.5-cm egg-shaped, Teflon-coated, magnetic stir bar, an internal thermocouple temperature probe, a 50-mL pressure-equalizing dropping funnel sealed with a rubber septum, and fitted with argon gas inlet.
The flask is flushed with argon and charged with benzyl bromoacetate (5) (13.8 g, 60.2 mmol, 1.00 equiv) and 300 mL of THF (Note 12).
The septum is removed temporarily and N,N'-ditosyl hydrazine (2) (30.8 g, 90.4 mmol, 1.50 equiv) is added.
The suspension is stirred in an ice bath while DBU (35.9 mL, 36.6 g, 241 mmol, 4.00 equiv) is added dropwise from dropping funnel over 5 min, such that the internal temperature does not exceed 20 °C (Note 13).
During the addition, the reaction mixture becomes homogenous and turns yellow.
The reaction mixture is stirred for 30 min and TLC analysis shows no starting material (Note 14).
Saturated aqueous sodium hydrogen carbonate solution (30 mL) is added and the reaction mixture is poured into Et2O (200 mL) and H2O (300 mL) in a 2-L separatory funnel.
The layers are separated, and the aqueous phase is extracted with an additional 2 × 200 mL of Et2O.
The organic solutions are combined, washed with 200 mL of brine, and dried over sodium sulfate (80 g).
Filtration through cotton plug, rinsing with 50 mL of Et2O and concentration on the rotary evaporator (30 °C, 37 mmHg, water bath) provides the crude mixture.
Ice-cooled Et2O (15 mL) is added and the white precipitates are removed in a fine-porosity sintered-glass Büchner funnel (diameter 90 mm) using suction filtration.
The precipitates are washed with ice-cooled Et2O (10 mL), and the filtrate is evaporated (30 °C, 37 mmHg) to give 11.1 g of the crude product as a clear, yellow oil.
This material is purified by silica gel column chromatography (elution with ethyl acetate/n-hexane) (Note 15, 16).
The combined fractions are concentrated by rotary evaporation (35 °C, 30 mmHg) and then at 25 °C, 4.0 mmHg for 3 h to furnish 8.56 g, (48.6 mmol, 81%) of benzyl diazoacetate (6) as a clear bright yellow oil (Note 17).
2. Notes
1.
When this procedure is run on smaller scale a Teflon-coated magnetic stirring bar can be used in place of the overhead mechanical stirrer.
2.
p-Toluenesulfonyl hydrazide (
1) (97%),
p-toluenesulfonyl chloride (99.0%), and HPLC grade CH
2Cl
2 (>99.8%) were purchased from Sigma-Aldrich and used as received.
The submitters purchased
p-toluenesulfonyl hydrazide (
1) (95%) and reagent grade CH
2Cl
2 (>99.0%) from Wako Pure Chemical Industries, Ltd., and the materials were used as received
p-Toluenesulfonyl chloride (99.0%) was purchased from Tokyo Chemical Industry Co., Ltd.
and the material was used as received.
3.
The consumption of the starting material
1 was monitored by TLC analysis on Merck silica gel 60 F
254 plates (0.25 mm, glass-backed, visualized with 254 nm UV lamp and stained with PMA) using 50% ethyl acetate in
n-hexane as an eluent.
p-Toluenesulfonyl hydrazide (
1) had R
f = 0.24 (weakly UV active, blue after staining).
4.
The addition of H
2O induces the formation of a white precipitate.
5.
A yield of 87% was observed when the reaction was performed on half scale.
The product displayed the following physicochemical properties: mp 226-228 ºC (decomp.);
1H NMR
pdf(DMSO-d
6, 600 MHz)
δ: 2.39 (s, 3 H), 7.38 (d,
J = 8.4 Hz, 2 H), 7.64 (d,
J = 8.4 Hz, 2 H), 9.58 (s, 1 H);
13C NMR
pdf(DMSO-d
6, 150 MHz) δ: 21.1, 127.8, 129.5, 135.5, 143.5; IR (neat, cm
−1) 3228, 3202, 1596, 1329, 1185, 1164, 1088, 813; HRMS Calcd.
for C
14H
17N
2O
4S
2 (M+H
+) 341.0624, Found 341.0624; Anal.
Calcd.
for C
14H
16N
2O
4S
2: C, 49.40; H, 4.74; N, 8.23; Found: C, 49.26; H, 4.62; N, 8.38.
6.
Sodium hydrogen carbonate (99.7%), dehydrated MeCN (99.8%), and benzyl alcohol (>99.0%) were purchased from Sigma-Aldrich and used as received.
The submitters purchased sodium hydrogen carbonate (>99.5%) and dehydrated MeCN (>99.0%) from Wako Pure Chemical Industries, Ltd., and benzyl alcohol (>99.0%) from Kanto Chemical Co., Inc.
All were used as received.
7.
Bromoacetyl bromide (98.0%) was purchased from Sigma-Aldrich and used as received.
The submitters purchased bromoacetyl bromide (98.0%) from Tokyo Chemical Industry Co., Ltd.
and used as received.
8.
The reaction was monitored by TLC analysis on Merck silica gel 60 F
254 plates (0.25 mm, glass-backed, visualized with 254 nm UV lamp and stained with PMA) using
n-hexane/EtOAc (6:1) as an eluent.
The product
5 had R
f = 0.53 (weakly UV active, dark brown after staining); benzyl alcohol had R
f = 0.18 (weakly UV active, black after staining).
9.
Silica gel was purchased from Silicycles Inc.
ultra pure 230-400 mesh silica gel, pH 7.0, H
2O content = 6%.
The submitters purchased silica gel (acidic) from Kanto Chemical Co., Inc.
(40-100 μm).
10.
The crude material is dissolved in eluent (5 mL) and then is charged onto a column (diameter = 7 cm, height = 5.5 cm) of 106 g (250 mL) of silica gel.
The column was eluted with
n-hexane /EtOAc (20:1) and 100-mL fractions were collected.
Fractions 2-20 were combined.
11.
A yield of 98% was observed when the reaction was performed on half scale.
Benzyl bromoacetate (
5) displayed the following physicochemical properties: IR (neat, cm
−1) 1732, 1273;
1H NMR
pdf(CDCl
3, 400 MHz)
δ: 3.88 (s, 2 H), 5.21 (s, 2 H), 7.35-7.39 (m, 5H);
13C NMR
pdf(CDCl
3, 100 MHz)
δ: 26.0, 68.0, 128.5, 128.7, 128.8, 135.1, 167.1; HRMS calcd for C
9H
9Br
1O
2Na
1 (M+Na
+) 250.9678, found 250.9676.
Anal.
Calcd.
for C
9H
9O
2Br
1: C, 47.19; H, 3.96; Found: C, 47.41; H, 4.11.
12.
THF (HPLC grade, H
2O = 0.003 %) was purchased from Fisher Scientific Company and distilled from sodium benzophenone ketyl.
13.
DBU (98%) was purchased from Sigma-Aldrich and used as received.
The submitters purchased DBU (98%) from Tokyo Chemical Industry Co., Ltd.
and used as received.
14.
The reaction was monitored by TLC analysis on Merck silica gel 60 F
254 plates (0.25 mm, glass-backed, visualized with 254 nm UV lamp and stained with PMA) using
n-hexane/EtOAc (6:1) as an eluent.
The product
6 had R
f = 0.44 (UV active, black after staining).
15.
Silica gel was purchased from Silicycles Inc.
ultra pure 230-400 mesh silica gel, pH 7.0, H
2O content = 6%.
The submitters purchased silica gel (neutral) from Kanto Chemical Co., Inc.
(40-100 μm).
16.
The crude material is dissolved in eluent (5 mL) and then is charged onto a column (diameter = 7 cm, height = 5.5 cm) of 106 g (250 mL) of neutral silica gel.
The column was eluted with
n-hexane/EtOAc (20:1) and 100-mL fractions were collected.
Fractions 5-30 were combined.
17.
A yield of 84% was observed when the reaction was performed on half scale.
Benzyl diazoacetate (
6) displayed the following physicochemical properties: IR (neat, cm
−1) 2105, 1683;
1H NMR
pdf(CDCl
3, 600 MHz)
δ: 4.80 (bs, 1 H), 5.20 (s, 2 H), 7.32-7.39 (m, 5 H);
13C NMR
pdf(CDCl
3, 150 MHz)
δ: 46.4, 66.5, 128.3, 128.4, 128.7, 136.0, 166.8; HRMS calcd for C
9H
8N
2O
2Na
1(M+Na
+) 199.0478, found 199.0476; Anal.
Calcd.
for C
9H
8N
2O
2: C, 61.36; H, 4.58; N, 15.90; Found: C, 61.43; H, 4.54; N, 15.79.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The use of diazocarbonyl compounds is becoming widespread by the advent of such important reactions as cyclopropanations and C-H insertion reactions as widely applicable and synthetically viable methods.
For the preparation of diazocarbonyl compounds, diazotransfer reaction is still the most conventional approach: the α-diazo group could be introduced into an ester or ketone
2 by treatment with sulfonyl azide.
Various modifications and improvements to this method have thus been developed to date towards milder reaction conditions and wider substrate scope.
2,3
Table 1. Substrate Scope.
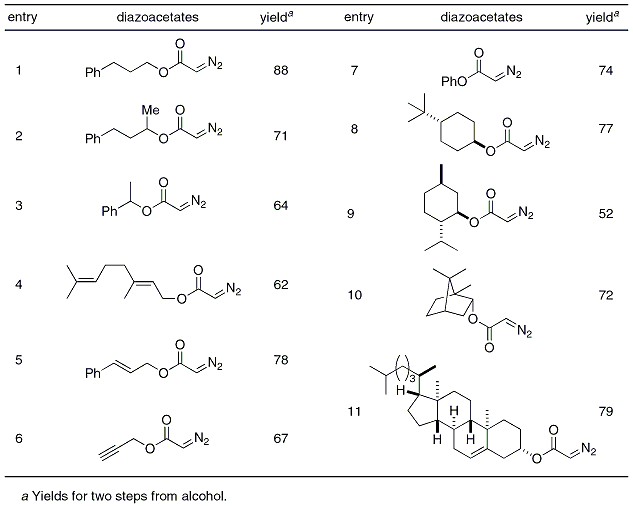
Nevertheless, prior introduction of a temporary activating group is necessary and sometimes become problematic, especially for the preparation of simple diazoacetates.
4 As House's procedure is one of the reliable alternatives
5 for the preparation of α-diazoacetates, this method requires the multi-step preparation of a corrosive and moisture-sensitive reagent: the
p-toluenesulfonylhydrazone of glyoxylic acid chloride.
The procedure presented here is a convenient preparative method for benzyl diazoacetate
6 from the corresponding bromoacetate and
N,
N'-ditosylhydrazine (
2), which can be applied to the preparation of various diazoacetates.
One-step synthesis of the air-stable reagent
2, the facile removal of the water-soluble wastes, and the facile availability of various bromoacetates make this methodology reliable as a safe and general synthetic procedure for the preparation of α-diazoacetates (Table 1).
α-Diazoketone could also be synthesized if the corresponding α-bromoketone is available.
A typical example is the synthesis of α-diazoacetophenone, which is often synthesized from benzoyl chloride and diazomethane.
The present method provides a one-step synthesis of α-diazoacetophenone, which circumvents the use of toxic and explosive diazomethane (Scheme 1).
The limitation of the method is the low reactivity toward secondary bromides, to which the sulfinate ion generated in situ attacks to afford a sulfone as a sole adduct.
Scheme 1. Synthesis of α-Diazoketone.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
p-Toluenesulfonyl hydrazide: Benzenesulfonic acid, 4-methyl-, hydrazide; (1576-35-8)
p-Toluenesulfonyl chloride: Benzenesulfonyl chloride, 4-mehyl-; (98-59-9)
N,N'-Ditosylhydrazine: Benzenesulfonic acid, 4-methyl-, 2-[(4-Methylphenyl)sulfonyl]hydrazide; (14062-05-6)
Sodium hydrogen carbonate: Carbonic acid sodium salt (1:1); (463-79-6)
Benzyl alcohol: Benzenemethanol; (100-51-6)
Bromoacetyl bromide: Acetyl bromide, 2-bromo-; (598-21-0)
Benzyl bromoacetate: Acetic acid, 2-bromo-, phenylmethyl ester; (5437-45-6)
DBU: Pyrimido[1,2-a]azepine, 2,3,4,6,7,8,9,10-octahydro-; (6674-22-2)
Benzyl diazoacetate: Acetic acid, 2-diazo-, phenylmethyl ester; (5226
7-51-3)
 |
Tohru Fukuyama received his Ph.D.
in 1977 from Harvard University with Yoshito Kishi.
He remained in Kishi's group as a postdoctoral fellow until 1978 when he was appointed as Assistant Professor of Chemistry at Rice University.
After seventeen years on the faculty at Rice, he returned to his home country and joined the faculty of the University of Tokyo in 1995, where he is currently Professor of Pharmaceutical Sciences.
He has primarily been involved in the total synthesis of complex natural products of biological and medicinal importance.
He often chooses target molecules that require development of new concepts in synthetic design and/or new methodology for their total synthesis. |
 |
Eiji Ideue was born in Kagawa, Japan in 1986.
He received his B.S.
in 2009 from the University of Tokyo.
In the same year, he began his graduate studies at the Graduate School of Pharmaceutical Sciences, the University of Tokyo, under the guidance of Professor Tohru Fukuyama.
His research interests are in the area of the total synthesis of natural products. |
 |
Tatsuya Toma was born in 1984 in Saitama, Japan.
He graduated in 2007 and received his M.
S.
degree in 2009 from the University of Tokyo.
The same year he started his Ph.
D.
study under the supervision of Professor Tohru Fukuyama.
His current interest is enantioselective total synthesis of complex natural products. |
 |
Jun Shimokawa was born in 1980 in Tokyo.
He performed his Ph.D.
studies under the direction of Professor Tohru Fukuyama at the University of Tokyo, where he conducted research on total syntheses of complex natural products.
In 2006, he was appointed Assistant Professor in the same group.
His research efforts focus on the development of novel synthetic methodology and applications to the synthesis of complex molecules. |
 |
Pablo E.
Guzmán was born in Aguascalientes, Mexico.
He earned his B.Sc.
in Chemistry from Chicago State University in 2006.
He is currently a doctoral student under the guidance of Professor Huw M.
L.
Davies.
His research is focused on the development of Rh(II)-catalyzed asymmetric vinylogous [4+3] cycloaddition reactions and the exploration of cascade bis-diazo methodology directed towards complexity-generating reactions |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved