It has been well-documented that a properly designed phosphine ligand is the key to advance cross-coupling processes. By strategically manipulating the phosphine ligand skeleton and thereby the geometry of the corresponding metal complexes, numerous transition metal-catalyzed cross-coupling processes have been successfully developed.
2 Various research groups have designed and synthesized supporting ligands to tackle challenging coupling reactions, such as P
t-Bu
3,
3 Buchwald ligands,
4 Josiphos,
5 Tang ligands,
6 Beller ligands,
7 Zhang ligands
8 and etc.
9 Despite the advancement in developing a variety of phosphine ligands, the rapid assembly of a structurally diverse ligand library
via simple synthetic methods remains essential for creating versatile catalysts for broader applications in coupling reactions.
Our research group has been engaged in developing several series of indolylphosphine ligands,
10 of which
CM-phos has proven to be an excellent ligand for Pd-catalyzed C-C bond and C-N bond formation.
11 The strategic considerations for designing such indolylphosphine ligands for coupling reactions include: (1) the use of inexpensive and readily available starting materials (i.e. arylhydrazine and acetophenone); (2) a simple and straightforward synthetic pathway (i.e. Fischer Indolization); (3) the ability to achieve a high level of steric and electronic fine-tuning for ligand diversity (Figure 1). Based on the above strategies, the
CM-phos scaffold was selected as a model template to design and explore a new series of phosphine ligands and their applications in Pd-catalyzed coupling reactions were investigated.
Figure 1. Ligand Design and Diversity.
Modification of the CM-phos at the Phosphorus Atom
Scheme 1. Synthetic Pathway of Pi-Pr-CM-phos and PPh-CM-phos.
In 2015, the direct C-3 arylation of imidazo[1,2-
a]pyridines with aryl tosylates was demonstrated.
12 By replacing the dicyclohexyl groups on the phosphorus atom of
CM-phos with the diisopropyl groups (Scheme 1), the catalytic performance significantly improved in the arylation reaction (i.e.
CM-phos: 77%
vs Pi-Pr-CM-phos: 88%). Particularly, this Pd/
Pi-Pr-CM-phos catalyst system enabled the first successful example of the coupling between aryl mesylate and imidazo[1,2-
a]pyridine (Scheme 2).
Scheme 2. Palladium-Catalyzed Direct Arylation of Imidazo[1,2-a]pyridines with Aryl Sulfonates.
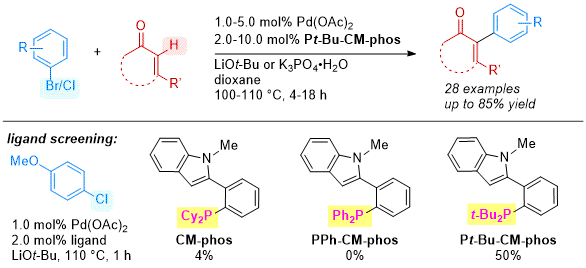
Scheme 3. Synthetic Pathway of Pt-Bu-CM-phos.
Recently, a new ligand,
Pt-Bu-CM-phos was prepared through a slightly modified phosphination process (Scheme 3). Instead of using
n-BuLi for a lithiation and subsequent trapping with chlorodialkylphosphine, magnesium was firstly added to react with the ligand precursor to form the Grignard reagent and followed by the addition of CuCl and di-
tert-butylchlorophosphine to afford the
Pt-Bu-CM-phos in 23% yield. The Pd/
Pt-Bu-CM-phos catalyst system was then employed in a regioselective palladium-catalyzed α-arylation of isophorone with 4-chloroanisole, achieving a 50% desired product yield (Scheme 4).
13 Neither
PPh-CM-phos (Scheme 1) nor
CM-phos can promote the reaction effectively, demonstrating the importance of the bulky
tert-butyl group at the phosphorus atom in the ligand scaffold. Through the study of kinetic isotopic effect, it was observed that the reaction rate of α-arylation reaction with D8-isophorone was slower than that of the H8-isophorone (kH8-IP/kD8-IP = 1.7). A control experiment using D5-isophorone as the coupling partner indicated that the reaction does not involve a C-H activation pathway but an isomerization process.
Scheme 4. Palladium-Catalyzed α-Arylation of Isophorone with Aryl Halides.
Modification of the CM-phos at the 2-Aryl Segment
Scheme 5. Synthetic Pathway of MeO-CM-phos, NMe2-CM-phos and (MeO)2-CM-phos.
In general, oxidative addition is favored by electron-rich phosphine ligands, which promotes the electron density at the metal center. In 2011, we designed a more electron-rich phosphine ligand by introducing a methoxy group at the
para-position to the -PCy
2 moiety on the
CM-phos scaffold (i.e.
MeO-CM-phos, Scheme 5), to facilitate the oxidative addition of aryl sulfonates in C-B bond coupling (Scheme 6).
14 Notably, the first successful borylation of aryl mesylates and tosylates was achieved using this Pd/
MeO-CM-phos catalyst system. The more electron-rich phosphine ligand enabled the coupling of less reactive aryl sulfonates more efficiently than the original Pd/
CM-phos catalyst system (i.e. 96%
vs 86% yield respectively).
Scheme 6. Pd-Catalyzed Borylation of Aryl Mesylates and Tosylates.
The same catalyst system can also be employed in the construction of C-N bonds between
NH-sulfoximines and aryl/alkenyl tosylates or aryl mesylates (Scheme 7).
15 Good functional group compatibility and good-to-excellent product yields were exhibited. Dialkylsulfoximines were also feasible coupling partners under this catalyst system. It is notable that the electron-rich Pd/
MeO-CM-phos resulted in a better catalytic efficacy than the previously reported Pd/
CM-phos catalyst system (i.e. 94%
vs 89% yield respectively).
Scheme 7. Pd-Catalyzed N-arylation of Sulfoximines with Aryl Sulfonates and Alkenyl Tosylates.
Pd-catalyzed cross-coupling reaction between organotitanium reagents and aryl sulfonates was demonstrated in 2020. By introducing an even more electron-donating dimethylamino group at the
para position of the -PCy
2-containing arene (
NMe2-CM-phos, Scheme 5), the oxidative addition process of inert C(Ar)-O bonds was further enhanced such that the
NMe2-CM-phos gave the best catalytic performance when compared to ordinary
CM-phos and
MeO-CM-phos (Scheme 8) (i.e. 46%
vs 16%
vs 23% respectively).
16 It is noteworthy that the catalyst loading can be down to 0.2 mol % and the reaction time can be shortened to 10 minutes.
Scheme 8. Pd-Catalyzed Cross-Coupling of Aryl Titanium and Aryl/Alkenyl Sulfonates.
In 2020, the first general palladium-catalyzed mono-
N-arylation of arylhydrazines with aryl tosylates was reported.
17 A more electron-enriched version of
MeO-CM-phos was prepared by attaching one more methoxy group to the phenyl ring on the ligand skeleton (
(MeO)2-CM-phos, Scheme 5). The
(MeO)2-CM-phos was shown to be more effective than the corresponding
CM-phos and
MeO-CM-phos in dealing with this arylation process (Scheme 9) (i.e. 85%
vs 73%
vs 80% respectively).
Scheme 9. Pd-Catalyzed Mono-N-arylation of Arylhydrazines with Aryl Tosylates.
Modification of the CM-phos at the Indole Segment
Scheme 10. Synthetic Pathway of 4,7-Me2-CM-phos.
The ï¬rst general palladium-catalyzed mono-α-arylation of aryl- and heteroarylketones with aryl mesylates and tosylates was explored in 2016 (Scheme 11).
18 Previous studies had shown that electron-rich ligands tend to be ineffective against electron-deficient arene substrates in ketone arylation reactions, as reported in the literature.
19 Therefore, turning attention from adjusting electronic properties to steric hindrance, the
4,7-Me2-CM-phos was specially designed by installation of two methyl groups at the 4,7-position of the indole segment of
CM-phos scaffold (Scheme 10) to enhance the steric congestion for facilitating reductive elimination in ketone arylation reactions. Excellent chemo- and monoselectivity with a modest catalyst loading (0.25-2.5 mol %) were attained under the Pd/
4,7-Me2-CM-phos catalyst system.
Scheme 11. Pd-Catalyzed Selective Mono-α-arylation of Acetophenone Enolate with Aryl Sulfonates.
Summary
A series of novel indolylphosphine ligands has been developed through targeted modifications of CM-phos at the phosphorus atom, 2-aryl segment and the indole segment. Such structural modifications have led to enhanced optimization of cross-coupling reactions, facilitating the use of lower catalytic loadings and accommodating a wider array of substrates. By increasing the electronic richness of CM-phos, challenging couplings involving aryl tosylates and mesylates as electrophilic coupling partners has been successfully achieved. Furthermore, the strategic addition or substitution of bulky groups within the ligand scaffold significantly promotes the reductive elimination process, thereby improving the overall catalytic efficacy. These fine-tunings on steric and electronic properties allow the newly developed ligands to have remarkable performance across various cross-coupling reactions, particularly in the formation of C-C bonds and C-N bonds.
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved