Org. Synth. 1985, 63, 99
DOI: 10.15227/orgsyn.063.0099
(2SR,3SR)-2,4-DIMETHYL-3-HYDROXYPENTANOIC ACID
[Pentanoic acid, 3-hydroxy-2,4-dimethyl-, (R*,R*)-]
Submitted by Stephen H. Montgomery, Michael C. Pirrung, and Clayton H. Heathcock
1.
Checked by Pauline J. Sanfilippo and Andrew S. Kende.
1. Procedure
A. 2,6-Dimethylphenyl propanoate. To a 2-L, three-necked, round-bottomed flask is added 26.4 g (0.55 mol) of a 50% dispersion of sodium hydride in mineral oil (Note 1). The sodium hydride is washed several times by decantation with dry hexane and is then covered with 1 L of dry ether (Note 2). The flask is immersed in an ice bath and equipped with a dropping funnel, a mechanical stirrer, and a reflux condenser. A solution of 61.1 g (0.50 mol) of 2,6-dimethylphenol (Note 3) in 150 mL of dry ether is added dropwise over a 10-min period and the mixture is stirred for 5 min, during which time hydrogen evolution ceases. The cold solution is stirred continuously while a solution of 48 mL (50.9 g, 0.55 mol) of propanoyl chloride (Note 1) in 100 mL of dry ether is added dropwise over a 30-min period. After stirring for an additional hour the reaction mixture is poured into a 2-L separatory funnel containing 200 mL of water. The mixture is shaken vigorously and the ether layer is separated and washed successively with 200 mL of aqueous 10% sodium hydroxide, 200 mL of water, and 200 mL of 4% hydrochloric acid, then dried over magnesium sulfate. The ether is removed with a rotary evaporator and the residue distilled through a short, indented Claisen apparatus to obtain 85–86 g (96–97%) of 2,6-dimethylphenyl propanoate, bp 60–65°C (0.05 mm) (Note 4).
B. 2',6'-Dimethylphenyl (2SR,3SR)-2,4-dimethyl-3-hydroxypentanoate. The reaction is carried out in a 2-L, three-necked, round-bottomed flask equipped with an efficient mechanical stirrer, a thermometer, and a 500-mL, pressure-equalizing dropping funnel. The dropping funnel is marked to hold 325 mL and is topped with a rubber septum pierced with a syringe needle attached to a source of dry nitrogen. The flask is charged with 300 mL of dry tetrahydrofuran (Note 2) and 69 mL (0.49 mol) of diisopropylamine (Note 1). Butyllithium (325 mL, 0.49 mol, 1.5 M in hexane) (Note 5) is transferred into the addition funnel with a cannula. The reaction flask and its contents are cooled to below −5°C by immersion in a bath of dry ice and isopropyl alcohol, which is maintained at −10 to −15°C by periodic additions of dry ice. The butyllithium is added dropwise at such a rate as to maintain the temperature of the reaction mixture in the range 0 to −5°C. After the addition is complete the mixture is stirred for an additional 15 min and is then cooled to −70°C. While the reaction mixture is cooling, the septum is briefly removed and a solution of 85 g (0.48 mol) of 2,6-dimethylphenyl propanoate in 100 mL of dry tetrahydrofuran is added to the addition funnel, the septum is replaced, and nitrogen is passed through the apparatus in a slow stream for 5 min. The ester is then added to the lithium diisopropylamide solution at such a rate that the temperature of the reaction mixture does not exceed −65°C. The total addition time is 30–40 min. After the addition is complete the reaction mixture is kept at −70°C for an additional hour, during which time the dropping funnel is charged with a solution of 35.3 g (0.49 mol) of 2-methylpropanal (Note 1) in 100 mL of dry tetrahydrofuran. The aldehyde solution is added dropwise to the vigorously stirring enolate solution at such a rate as to maintain a reaction temperature of less than −65°C. After the addition is complete the reaction mixture is kept at −70°C for an additional 30 min. To the vigorously stirring solution is added 500 mL of saturated aqueous ammonium chloride. At this point stirring is discontinued, the cooling bath is removed, and the partially frozen mixture is allowed to warm to room temperature. The contents of the reaction flask are introduced into a large separatory funnel and diluted with 500 mL of ether. The layers are separated, and the organic phase is washed with 300 mL of water and 300 mL of saturated brine and then dried over magnesium sulfate. After removal of the drying agent the solvents are removed with a rotary evaporator to give 112–120 g of an oily semisolid, which is a 7 : 2 mixture of the β-hydroxy ester and 2,6-dimethylphenyl propanoate. This material may be crystallized from ether–hexane to provide 70 g (60%) of pure β-hydroxy ester, mp 75.5–76°C (Note 6). However, it is not necessary to purify the crude product before hydrolysis to the β-hydroxy acid (Note 7).
C. (2SR,3SR)-2,4-Dimethyl-3-hydroxypentanoic acid. The crude product from the foregoing preparation (112–120 g) is dissolved in 500 mL of methanol and placed in a 2-L Erlenmeyer flask. A solution of 112 g (2 mol) of potassium hydroxide in a mixture of 500 mL of water and 500 mL of methanol is added with stirring, whereupon the reaction mixture warms to about 40°C. After stirring for 15 min crushed dry ice is added in portions to the vigorously stirring mixture until the pH is 7–8. The resulting solution is concentrated to a volume of about 500 mL with a rotary evaporator and extracted with two 300-mL portions of methylene chloride, which are discarded. The aqueous phase is then acidified to pH 1–2 by addition of 75 mL of concentrated hydrochloric acid (vigorous evolution of CO2) and extracted with two 500-mL portions of methylene chloride. The combined organic extracts are washed with 200 mL of saturated brine and dried over magnesium sulfate. After removal of the drying agent the solvent is removed with a rotary evaporator to obtain 36–53 g of (2SR,3SR)-2,4-dimethyl-3-hydroxypentanoic acid as a semisolid. Crystallization from hexane provides 30–43 g (41–60% overall yield) of pure hydroxy acid, mp 76–79°C (Note 8).
2. Notes
1.
Sodium hydride was obtained from Ventron Corporation, Morton Thiokol Inc. 2,6-Dimethylphenol and propanoyl chloride were obtained from Aldrich Chemical Company, Inc. and used without further purification.
Diisopropylamine was distilled from
calcium hydride prior to use.
2-Methylpropanal was distilled prior to use.
2.
Reagent-grade diethyl ether from a freshly opened
container was used without further purification.
Reagent-grade tetrahydrofuran was dried over
sodium before use.
3.
2,6-Dimethylphenol is a corrosive, poisonous substance that is readily absorbed through the skin. All reactions should be carried out in an
efficient hood, and appropriate protective apparel should be used.
4.
The IR spectrum (neat) shows an absorption at 1755 cm
−1. The
1H NMR spectrum (CDCl
3) is as follows δ: 1.27 (t, 3 H,
J = 7), 2.13 (s, 6 H), 2.55 (q, 2 H,
J = 7), 6.90 (s, 3 H).
5.
Butyllithium was obtained from Foote Mineral Company, Johnsonville, Tennessee. It may be standardized by a double-titration procedure.
26.
The IR spectrum (neat) has absorptions at 3500 and 1750 cm
−1. The
1H NMR spectrum is as follows δ: 1.00 (d, 3 H,
J = 7), 1.07 (d, 3 H,
J = 7), 1.40 (d, 3 H,
J = 7), 2.20 (s, 6 H), 2.93 (quintet 1 H,
J = 7), 3.50 (m, 2 H), 7.03 (s, 3 H).
7.
The checkers found that hydrolysis of once-crystallized aldol (mp 74–75°C) gives a hydroxy acid that crystallizes readily from
hexane, for an overall two-step yield of
32%. Hydrolysis of the crude aldol product gives the hydroxy acid as an oil that crystallizes with difficulty, for an overall two-step yield of
45%.
8.
The IR spectrum (neat) has absorptions at 3500, 3300–2500, and 1695 cm
−1. The
1H NMR spectrum is as follows δ: 0.93 (d, 3 H
J = 7) 0.99 (d, 3 H,
J = 7), 1.24 (d, 3 H,
J = 7), 1.81 (octet, 1 H,
J = 6), 2.69 (quintet, 1 H,
J = 7), 3.44 (t, 1 H,
J = 5.6), 7.4 (br s, 2 H, OH).
3. Discussion
A number of methods have been developed for accomplishing aldol addition reactions in a stereoselective manner.
3 4 5 The performed lithium enolates of alkyl esters normally react with aldehydes to give mixtures of the two diastereomeric β-hydroxy esters:
6However, the enolates derived from certain aryl esters add to aldehydes to give largely one stereoisomeric product:
7 8The aryl groups that have been investigated are 2,6-dimethylphenyl (DMP), 2,6-di-tert-butyl-4-methylphenyl (BHT), and 2,6-di-tert-butyl-4-methoxyphenyl (DBHA). Selected examples are shown in Table 1. The most convenient reagents, because of the ease of their further manipulations, are the DMP esters. With aliphatic aldehydes branched at the α-carbon, the DMP esters give essentially one diastereomeric product, β-hydroxy ester 1. With aromatic and α-unbranched aliphatic aldehydes, the DMP esters give predominantly, but not entirely, one isomer. In these cases the BHT or DBHA esters may be used. Acrolein gives a mixture of 1 and 2 even with the BHT and DBHA esters.
Aryl esters of other acids show similar stereoselectivity; examples are as follows:
In addition, the
BHT esters of O-benzyllactic acid condense with aldehydes to give diastereomerically homogeneous adducts:
9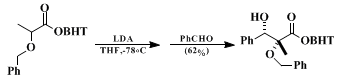
TABLE I
CONDENSATION OF ARYL ESTERS WITH ALDEHYDES
|
|
Ar Ester
|
R
|
1 : 2
|
mp (°C)
|
Yield (%)a
|
|
|
DMP
|
C6H5—
|
72
|
88/12
|
62–63c
|
|
DMP
|
n-C5H11
|
70
|
86/14
|
Oil
|
|
DMP
|
i-C3H7—
|
78
|
>98/2
|
76
|
|
DMP
|
t-C4H9—
|
82
|
>98/2
|
70–71
|
|
DMP
|
C6H5(CH3)CH—
|
81
|
>98/2
|
Oild
|
|
BHT
|
CH2=CH—
|
88
|
85/15
|
64–67e
|
|
BHT
|
CH2=C(CH3)—
|
88
|
>98/2
|
70–71
|
|
BHT
|
C6H5—
|
96
|
>98/2
|
Oil
|
|
BHT
|
i-C3H7—
|
100b
|
>98/2
|
105–106
|
|
BHT
|
C6H5(CH3)CH—
|
100b
|
>98/2
|
Oild
|
|
DBHA
|
CH2=CH—
|
90
|
87/13
|
65–72f
|
|
DBHA
|
C6H5—
|
75
|
>98/2
|
59–61
|
|
DBHA
|
n-C5H11—
|
70
|
>98/2
|
Oil
|
|
DBHA
|
i-C3H7—
|
79
|
>98/2
|
91–93
|
|
DBHA
|
t-C4H9—
|
77
|
>98/2
|
88–89
|
|
aAll reactions were carried out on a 1-mmol scale. Unless otherwise noted, yields are for high-performance liquid chromatography (HPLC)-purified product. On a larger scale, such as is given in this procedure, yields are somewhat lower.
|
bThis is the yield of crude product; these products were not purified by chromatography.
|
cMelting point given is that of the major diastereomer (1).
|
dMixture of Cram's rule and anti-Cram's rule diastereomers: ratio = 4 : 1.
|
eMelting point given is for a 95 : 5 mixture of 1 : 2.
|
fMelting point given is for a 90 : 10 mixture of 1 : 2.
|
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Pentanoic acid, 3-hydroxy-2,4-dimethyl-, (R*,R*)-
(2SR,3SR)-2,4-Dimethyl-3-hydroxypentanoic acid
2',6'-Dimethylphenyl (2SR,3SR)-2,4-dimethyl-3-hydroxypentanoate
2,6-dimethylphenyl (DMP)
2,6-di-tert-butyl-4-methylphenyl (BHT)
2,6-di-tert-butyl-4-methoxyphenyl (DBHA)
BHT esters of O-benzyllactic acid
hydrochloric acid (7647-01-0)
methanol (67-56-1)
ether,
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
hydrogen (1333-74-0)
sodium hydroxide (1310-73-2)
Acrolein (107-02-8)
nitrogen (7727-37-9)
CO2 (124-38-9)
potassium hydroxide (1310-58-3)
sodium (13966-32-0)
methylene chloride (75-09-2)
magnesium sulfate (7487-88-9)
propanoyl chloride (79-03-8)
2-methylpropanal (78-84-2)
butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
sodium hydride (7646-69-7)
hexane (110-54-3)
calcium hydride (7789-78-8)
2,6-dimethylphenol (576-26-1)
lithium diisopropylamide (4111-54-0)
diisopropylamine (108-18-9)
2,6-Dimethylphenyl propanoate (51233-80-8)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved