All organic chemists have experienced frustration at one time or another when attempting to repeat reactions based on experimental procedures found in journal articles. To ensure reproducibility, Organic Syntheses requires experimental procedures written with considerably more detail as compared to the typical procedures found in other journals and in the "Supporting Information" sections of papers. In addition, each Organic Syntheses procedure is carefully "checked" for reproducibility in the laboratory of a member of the Board of Editors.
Even with these more detailed procedures, the experience of Organic Syntheses editors is that difficulties often arise in obtaining the results and yields reported by the submitters of procedures. To expedite the checking process and ensure success, we have prepared the following "Instructions for Authors" as well as a
Checklist for Authors and
Characterization Checklist
to assist you in confirming that your procedure conforms to these requirements.
Please include completed Checklists together with your procedure at the time of submission. Procedures submitted to Organic Syntheses will be carefully reviewed upon receipt and procedures lacking any of the required information will be returned to the submitters for revision.
Unless otherwise indicated, these instructions apply to articles that describe chemical reactions as well as to OS Techniques articles that focus on experimental techniques. Not all of these instructions will be relevant in the case of some OS Techniques articles and authors are encouraged to contact the Editor in Chief and the Associate Editor with any questions.
The appropriate scale for procedures and to illustrate techniques will vary depending on the nature of the chemistry and the compounds synthesized in the procedure. However, some general guidelines are possible.
Scale: Chemical Reactions
For procedures in which the principal goal is to illustrate a synthetic method or strategy, it is expected, in general, that the procedure should result in at least 2 g and no more than 50 g of the final product. In cases where the point of the procedure is to provide an efficient method for the preparation of a useful reagent or synthetic building block, the appropriate scale also should be between 2 and 50 g of final product. Exceptions to these guidelines may be granted in special circumstances. For example, procedures describing the preparation of reagents employed as catalysts will often be acceptable on a scale of less than 2 g.
Scale: OS Techniques
The scale in OS Techniques articles will vary and may involve relatively small amounts of compounds depending on the nature of the technique. Authors should consult with the Editor in Chief and Associate Editor for guidance when they have questions concerning the appropriate scale to demonstrate a technique.
Scale and Costs
In considering the scale for all Organic Syntheses procedures, authors should also take into account the cost of reagents and starting materials. In general, the Editors will not accept procedures for checking in which the cost of any one of the reactants exceeds $500 for a single full-scale run. Authors are required to identify the most expensive reagent or starting material on the procedure submission checklist and to estimate its cost per run of the procedure. Authors should purchase chemicals from common suppliers that are well known to the chemistry community. It is also expected that the cost will not exceed $500 for any unusual equipment not found in most chemistry laboratories.
Optimization
It is expected that all aspects of the procedure or technique will have been optimized by the authors prior to submission, and it is required that each reaction or experimental operation will have been carried out at least twice on exactly the scale described in the procedure, and with the results reported in the manuscript. It is recommended that the reaction(s) and operations be tested by a coworker not been involved in the project as an "internal check" that the experimental procedures are clear and unambiguous and that the chemistry is reproducible in the hands of a researcher without prior experience in the area.
It is appropriate to report the weight, yield, and purity of the product of each step in a procedure as a range. Yields should be rounded off to the nearest percent and purity if measured by qNMR should be reported to tenths of a percent. A range in yield over several runs of more than 10% may indicate that there are variables affecting the reaction that have not been identified and this may be cause for rejection of the procedure.
In any case where a reagent is employed in significant excess (e.g., more than 1.5 equiv), a Note should be included explaining why an excess of that reagent is necessary. If possible, the Note should indicate the effect of using amounts of reagent less than that specified in the procedure.
A unique feature of papers published in Organic Syntheses is that each procedure and all characterization data is carefully checked for reproducibility in the laboratory of a member of the Board of Editors. At least two successful runs of each step must be completed, at least one of which must be on exactly the author's scale, and the other(s) on the author's scale or on no less than half of that scale. In general, the yields and levels of selectivity must be no less than ca. 5% below that reported by the authors. If problems are encountered, then the checking editor will contact the authors for advice. In the event that an editor finds it necessary to make any modifications in an experimental procedure, then the published article incorporates the modified procedure, with an explanation and mention of the original protocol often included in a Note. The yields reported in the published article are always those obtained by the checkers. In general, the characterization data in the published article also is that of the checkers, unless there are significant differences with the data obtained by the authors, in which case the author's data will also be reported in a Note.
To ensure reproducibility, Organic Syntheses requires experimental procedures written with considerably more detail as compared to the typical procedures found in other journals and in the “Supporting Information” sections of papers. Authors should carefully review the following instructions in preparing their articles for submission. A list of “common omissions” outlines some of the issues identified by the editors in recently submitted articles; full details on the relevant instructions in each case can be found in the main sections following this summary outline.
Common Omissions
- A full description is required for all reaction setups, including how each neck of flasks are equipped (this is in addition to the photos of the setup).
- Do not use balloons for maintaining an inert atmosphere in reactions.
- Room temperature and vacuum pressures must be defined explicitly.
- If a product is used in the next step without purification, then a Note is required describing the purification of a sample and characterization data for both the purified sample and the crude material used in the next step is required.
- The amount of product generated in a prior step must be more than or equal to the amount used as starting material in a subsequent step.
- Yields must be rounded off to the nearest percent.
- Significant figures must be used correctly.
- When qNMR is employed to determine purity, then a Note must be included reporting the identity of the internal standard that was used and the copies of the spectra must have the calculations printed on them.
Describe the size and type of flask (number of necks) and indicate how every neck is equipped.
"A 500-mL, three-necked, round-bottomed flask equipped with a 3-cm Teflon-coated magnetic stirbar, a 250-mL pressure-equalizing addition funnel fitted with an argon inlet, and a rubber septum is charged with . . . ."
Indicate how the reaction apparatus is dried and whether the reaction is conducted under an inert atmosphere. Note that in general balloons are not acceptable as a means of maintaining an inert atmosphere unless warranted by special circumstances. For reactions conducted below -20 °C it is advisable to include a thermocouple or thermometer to monitor the internal temperature of the reaction mixture.
Further details concerning the reaction apparatus can be incorporated in the text of the procedure or included in a Note.
"The apparatus is flame-dried and maintained under an atmosphere of argon during
the course of the reaction."
Photographs are required depicting the reaction apparatus and in the case of procedures involving unusual glassware or especially complicated reaction setups, authors may wish to include a drawing of the apparatus in the text or in a Note (for example, see Org. Synth. 2016, 93, 127-145).
Use of Gloveboxes
When a glovebox is employed in a procedure, justification must be provided in a Note and the consequences of carrying out the operation without using a glovebox must be discussed.
Reagents and Starting Materials
All chemicals employed in the procedure must be commercially available or their synthesis must be described in an earlier Organic Syntheses or Inorganic Syntheses procedure. For other compounds, a procedure should be included either as one or more steps in the text or, in the case of relatively straightforward preparations of reagents, as a Note. In the latter case, all requirements with regard to characterization, style, and detail also apply. In general, the Editors will not accept procedures for checking in which the cost of any one of the reactants exceeds $500 for a single full-scale run. Authors are encouraged to consult with the Associate Editor if they have any question as to whether to include the preparation of any chemical as part of the text or as a Note.
Authors are encouraged to consider the use of "substitute solvents" in
place of more hazardous alternatives. For example, the use of t-butyl methyl ether (MTBE) should be considered as a substitute for diethyl
ether, particularly in large scale work. Authors are referred to the
articles "Sanofi's Solvent Selection Guide: A Step Toward More Sustainable
Processes" (Prat, D.; Pardigon, O.; Flemming, H.-W.; Letestu, S.; Ducandas,
V.; Isnard, P.; Guntrum, E.; Senac, T.; Ruisseau, S.; Cruciani, P. Hosek,
P. Org. Process Res. Dev. 2013, 17, 1517-1525) and "Solvent Replacement for Green Processing" (Sherman, J.;
Chin, B.; Huibers, P. D. T.; Garcia-Valis, R.; Hatton, T. A. Environ. Health Perspect. 1998, 106
(Supplement I, 253-271) as well as the references cited therein for
discussions of this subject. In addition, a link to a "solvent selection
guide" can be accessed via the American Chemical Society Green Chemistry
website at http://www.acs.org/content/acs/en/greenchemistry/research-innovation/tools-for-green-chemistry.html.
In one or more Notes, indicate the purity or grade of each reagent, solvent, etc. It is also expected that authors will indicate the source (company the chemical was purchased from), particularly in the case of chemicals where it is suspected that the composition (trace impurities, etc.) may vary from one supplier to another. In cases where reagents are purified, dried, "activated" (e.g., Zn dust), etc., a detailed description of the procedure used should be included in a Note. In other cases, indicate that the chemical was "used as received".
"Diisopropylamine (99.5%) was obtained from Aldrich Chemical Co., Inc. and distilled under argon from calcium hydride before use. THF (99+%) was obtained from Mallinckrodt, Inc. and distilled from sodium benzophenone ketyl. Diethyl ether (99.9%) was purchased from Aldrich Chemical Co., Inc. and purified by pressure filtration under argon through activated alumina. Methyl iodide (99%) was obtained from Aldrich Chemical Co., Inc. and used as received."
The amount of each reactant must be provided in parentheses in the order
mL, g, mmol, and equivalents with careful consideration to the correct
number of significant figures. Avoid indicating amounts of reactants
with more significant figures than makes sense. For example, "437 mL of
THF" implies that the amount of solvent must be measured with a level of
precision that is unlikely to affect the outcome of the reaction. Likewise,
"5.00 equiv" implies that an amount of excess reagent must be controlled to
a precision of 0.01 equiv.
The concentration of solutions should be expressed in terms of molarity or normality, and not percent (e.g., 1 N HCl, 6 M NaOH, not "10% HCl").
For chemical reactions, describe every aspect of the reaction procedure clearly and explicitly. Indicate the order of addition and time for addition of all reagents and how each is added (via syringe, addition funnel, etc.).
Indicate the temperature of the reaction mixture (preferably internal temperature). For reactions carried out at room temperature provide the temperature in °C (i.e., do not report as "rt"). Describe the type of cooling (e.g., "dry ice-acetone bath") and heating (e.g., oil bath, heating mantle) methods employed. Be careful to describe clearly all cooling and warming cycles, including initial and final temperatures and the time interval involved.
Describe the appearance of the reaction mixture (color, homogeneous or not, etc.) and describe all significant changes in appearance during the course of the reaction (color changes, gas evolution, appearance of solids, exotherms, etc.).
Indicate how the reaction can be monitored to determine the extent of conversion of reactants to products. In the case of reactions monitored by TLC, provide details in a Note, including eluent, Rf values, and method of visualization. In most cases it is desirable to include a photograph of the developed TLC plate. For reactions followed by GC, HPLC, or NMR analysis, provide details on analysis conditions and relevant diagnostic peaks.
"The progress of the reaction was followed by TLC analysis on silica gel
with 1:4 EtOAc-hexane as eluent and visualization with p-anisaldehyde. The ketone starting material has Rf = 0.40
(green) and the alcohol product has Rf = 0.25 (blue)."
Details should be provided for reactions in which a "quenching" process is involved. Describe the composition and volume of quenching agent, and time and temperature for addition. In cases where reaction mixtures are added to a quenching solution, be sure to also describe the setup employed.
"The resulting mixture was stirred at room temperature for 15 h, and then carefully poured over 10 min into a rapidly stirred, ice-cold aqueous solution of 1 N HCl in a 500-mL Erlenmeyer flask equipped with a magnetic stirbar (shape and size)."
For extractions, the number of washes and the volume of each should be indicated as well as the size of the separatory funnel.
For concentration of solutions after workup, indicate the method and pressure and temperature used.
"The reaction mixture is diluted with 200 mL of water and transferred to a
500-mL separatory funnel, and the aqueous phase is separated and extracted
with three 100-mL portions of ether. The combined organic layers are washed
with 75 mL of water and 75 mL of saturated NaCl solution, dried over 25 g
of MgSO4, filtered through a 250-mL medium porosity sintered glass funnel, and
concentrated by rotary evaporation (25 °C, 20 mmHg) to afford 3.25 g
of a yellow oil."
"The solution is transferred to a 250-mL, round-bottomed flask equipped with
a magnetic stirbar (shape and size) and a 15-cm Vigreux column fitted with a short path
distillation head, and then concentrated by careful distillation at 50 mmHg
(bath temperature gradually increased from 25 to 75 °C)."
In cases where solid products are filtered, describe the size, type, and porosity of the filter funnel and filter paper (if applicable) used and the amount and composition of solvents used for washes.
". . . and the resulting pale yellow solid is collected by filtration on a Büchner funnel and washed with 100 mL of cold (0 °C) hexane."
When solid or liquid compounds are dried under vacuum, indicate the
pressure employed (rather than stating "reduced pressure" or "dried in vacuo").
" . . . . and concentrated at room temperature by rotary evaporation (20 mmHg) and then at 0.01 mmHg to provide . . . . "
"The resulting colorless crystals are transferred to a 50-mL, round-bottomed
flask and dried overnight in a 100 °C oil bath at 0.01 mmHg."
Purification: Distillation
Describe distillation apparatus including the size and type of distillation column. Indicate temperature (and pressure) at which all significant fractions are collected.
" ....and transferred to a 100-mL, round-bottomed flask equipped with a
magnetic stirbar. The product is distilled under vacuum through a 12-cm,
vacuum-jacketed column of glass helices (Note 16) topped with a Perkin
triangle. A forerun (ca. 2 mL) is collected and discarded, and the desired
product is then obtained, distilling at 50-55 °C (0.04-0.07 mmHg) . .
. . "
Purification: Column Chromatography
Provide information on TLC analysis in a Note, including eluent, Rf values, and method of visualization.
Provide dimensions of column and amount of silica gel used; in a Note indicate source and type of silica gel.
Provide details on eluents used, the number and size of fractions, and which fractions were combined to provide the purified product.
"The product is charged on a column (5 x 10 cm) of 200 g of silica gel (Note
15) and eluted with 250 mL of hexane. At that point, fraction collection
(25-mL fractions) is begun, and elution is continued with 300 mL of 2%
EtOAc-hexane (49:1 hexanes:EtOAc) and then 500 mL of 5% EtOAc-hexane (19:1
hexanes:EtOAc). The desired product is obtained in fractions 24-30, which
are concentrated by rotary evaporation (25 °C, 15 mmHg)...."
Use of Automated Column Chromatography
Automated column chromatography should not be used for purification of products unless the use of such systems is essential to the success of the procedure. When automated column chromatography equipment is employed in a procedure, justification must be provided in a Note and the consequences of carrying out the purification using conventional column chromatography must be discussed.
Purification: Recrystallization
Describe the procedure in detail. Indicate solvents used (and ratio of mixed solvent systems), amount of recrystallization solvents, and temperature protocol. Describe how crystals are isolated and what they are washed with. A photograph of the crystalline product is often valuable to indicate the form and color of the crystals.
"The solid is dissolved in 100 mL of hot diethyl ether (30 °C) and filtered through
a Buchner funnel. The filtrate is allowed to cool to room temperature, and 20 mL
of hexane is added. The solution is cooled at -20 °C overnight and the resulting
crystals are collected by suction filtration on a Buchner funnel, washed with 50
mL of ice-cold hexane, and then transferred to a 50-mL, round-bottomed flask and
dried overnight at 0.01 mmHg to provide...."
If filter paper is employed in the filtration, the type of filter paper should be described..
Characterization and Proof of Purity
Physical properties of the product such as color, appearance, crystal forms, melting point, etc. should be included in the text of the procedure. Comments on the stability of the product to storage, etc. should be provided in a Note.
In a Note, provide data establishing the identity of the product. This will generally include IR (at least key absorptions), mass spec, 1H-NMR, and 13C-NMR data, and in some cases UV data. Copies of the proton and carbon NMR spectra for the products of each step in the procedure should be submitted showing integration for all resonances. Submission of copies of the NMR spectra for other nuclei are encouraged as appropriate.
In the same or a separate Note, provide analytical data establishing that the purity of the isolated product is at least 97%. Note that this data should be obtained for the material on which the yield of the reaction is based, not for a sample that has been subjected to additional purification by chromatography, distillation, or crystallization. Elemental analysis for carbon and hydrogen (and nitrogen if present) agreeing with calculated values within 0.4% is preferred. However, quantitative NMR (qNMR), GC, or HPLC analyses involving measurements versus an internal standard will also be accepted. See Appendix I of these instructions for procedures for quantitative analysis of purity by NMR and chromatographic methods. Authors must provide details on equipment and conditions for GC and HPLC analyses, and copies of NMR spectra and chromatograms used in the quantitative analyses must be submitted with articles for publication. When qNMR is used to establish purity, then the details of the analysis (e.g., amounts of the compound and the internal standard) and the calculations must be printed on the copy of the NMR spectrum. All spectra should be provided as individual pages within the PDF document.
In procedures involving non-racemic, enantiomerically enriched products, optical rotations should generally be provided, but enantiomeric purity must be determined by another method such as chiral HPLC or GC analysis. A chromatogram of racemic material should also be provided as a reference standard.
In cases where the product of one step is used without purification in the next step, a Note should be included describing how a sample of the product can be purified and providing characterization data for the pure material. Copies of the proton NMR spectra of both the product both before and after purification should be submitted.
Safety Note and Hazard Warnings
The first Note in every article is devoted to addressing the safety aspects of the procedures described in the article. The Article Template provides the required wording and format for Note 1, which reminds readers of the importance of carrying out risk assessments and hazard analyses prior to performing all experiments:
Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with (enter list of chemicals here), as well as the proper procedures for (list any unusual experimental operations here). (Provide additional cautions with regard to exceptional hazards here).
For the required list of chemicals, authors should include all reactants, solvents, and other chemicals involved in the reactions described in the article.
With regard to the list of experimental operations, this list should be limited to those operations that potentially pose significant hazards. Examples may include
• Vacuum distillations
• Reactions run at elevated pressure or in sealed reaction vessels
• Photochemical reactions
In the case of experiments that involve exceptional hazards such as the use of pyrophoric or explosive substances, and substances with a high degree of acute or chronic toxicity, authors should provide additional guidelines for how to carry out the experiment so as to minimize risk. These instructions formerly would have appeared as red "Caution Notes" in Organic Syntheses articles. Note that it is not essential to describe general safety procedures such as working in a hood, avoiding skin contact, using eye protection, etc., since these are discussed in the Prudent Practices reference mentioned in the "Working with Hazardous Chemicals" statement within each article.
Efforts should be made to avoid the use of toxic and hazardous solvents and reagents when less hazardous alternatives are available.
The style and content of the discussion section will depend on the nature of the article.
For procedures that provide an improved method for the preparation of an important reagent or synthetic building block, the discussion should focus on the advantages of the new approach and should describe and reference all of the earlier methods used to prepare the title compound.
In the case of procedures that illustrate an important synthetic method or strategy, the discussion section should provide a mini-review on the new methodology. The scope and limitations of the method should be discussed, and it is generally desirable to include a table of examples. Please be sure each table is numbered and has a title. Competing methods for accomplishing the same overall transformation should be described and referenced. A brief discussion of mechanism may be included if this is useful for understanding the scope and limitations of the method.
The discussion section of OS Techniques articles will vary depending on the nature of the technique that is being illustrated in the article. In some cases it will be appropriate to discuss variations in the technique or to contrast the technique with alternative means of accomplishing the same purpose. Authors are referred to the discussion section of the inaugural OS Technique article as a model (Senzer, B. D.; Varongchayakul, C.; Danheiser, R. L.; Daniels, B.; Dong, V. M. Purification of Organic Compounds by Flash Column Chromatography. Org. Synth. 2025, 102, 276-302, DOI: 10.152271orgsyn.102.0276).
In cases where the main thrust of the article is the illustration of a technique or synthetic method of general utility, the title of the article should incorporate reference to that method. Inclusion of the name of the final product is acceptable but not required. In the case of articles where the objective is the preparation of a specific compound of importance (such as a chiral ligand), then the name of that compound should be part of the title.
Examples
Title without name of product:
"Stereoselective Synthesis of 3-Arylacrylates by Copper-Catalyzed Syn Hydroarylation"
(Org. Synth. 2010, 87, 53).
Title including name of final product (note name of product is not required):
"Catalytic Enantioselective Borane Reduction of Benzyl Oximes: Preparation of (S)-1-Pyridin-3-yl-ethylamine
Bis Hydrochloride" (Org. Synth. 2010, 87, 36).
Title where preparation of specific compound is the subject:
"Preparation of (S)-3,3'-Bis-Morpholinomethyl-5,5',6,6',7,7',8,8'-octahydro-1,1'-bi-2-naphthol"
(Org. Synth. 2010, 87, 59).
The title of the article is followed by a listing of the authors of the submitted article and their addresses. Contact information and other information for the principal author is included in footnote 1 (see below). This is followed by a listing of the Organic Syntheses junior checkers and Organic Syntheses checking editor and their address. Footnote 2 provides contact information for the Organic Syntheses editor.
The title of the article should be followed by a “Title Scheme” comprising separate equations for each step in the article. Authors should consult the article template for instructions concerning ChemDraw settings and format. In general, reaction equations should not include details such as reaction time and the number of equivalents of reagents, with the exception of reactants employed in catalytic amounts which can be labeled as “cat.” or by specifying mol%.
The title scheme for OS Techniques articles will vary depending on the nature of the technique and may incorporate illustration of equipment. Authors should consult with the Editor in Chief and Associate Editor for guidance when they have questions concerning what may be appropriate as the heading graphic for an OS Techniques article.
Style and Format for Text
The text of articles should follow the style guidelines used for organic chemistry articles published in the ACS journals such as J. Am. Chem. Soc., J. Org. Chem., Org. Lett., etc. as described in the ACS Style Guide (3rd Ed.). Present or past tense should be used in the description of procedures. The text of the procedure should be created using the Word template available on the Organic Syntheses website. Specific instructions with regard to the manuscript format (font, spacing, margins) is available on the website in the “Instructions for Article Template” and embedded within the Article Template itself.
Style and Format for Tables and Schemes
Chemical structures and schemes should be drawn using the standard ACS drawing parameters (in ChemDraw, the parameters are found in the "ACS Document 1996" option) with a maximum full size width of 15 cm (5.9 inches). The graphics files should then be pasted into the Word document at the correct location and the size reduced to 75% using "Format Picture" (Mac) or "Size and Position" (Windows). Graphics files must also be submitted separately. All Tables that include structures should be entirely prepared in the graphics (ChemDraw) program and inserted into the word processing file at the appropriate location. Tables that include multiple, separate graphics files prepared in the word processing program will require modification.
Tables and schemes should be numbered and should have titles. The title for a Table should be included immediately above the table. The title for a scheme should be placed immediately below the scheme. Use 10 point Palatino Bold font in the ChemDraw file for all titles. For footnotes in Tables use Helvetica (or Arial) 9 point font and place these immediately below the Table.
Style and Format for References in Footnotes
References to prior literature included as footnotes should follow the current style requirements of JACS and must include the titles of articles as well as DOIs using the format DOI:10.###/XXXX.XX.
Photographs illustrating key elements of procedures are required in every article published in Organic Syntheses. Authors are expected to incorporate photos in their original submissions and photos may also be provided by the Checkers of procedures. Photographs should be inserted into articles at the place in the text and Notes where they are first referred to and should be numbered and labeled as Figures with descriptive titles. Particularly useful subjects for photographs include:
• Photos of reaction flasks depicting how each neck is equipped
• Photos of reaction mixtures illustrating color changes, heterogeneity, etc.
• Photos of TLC plates showing degree of resolution and the color of spots
• Photos of crystalline reaction products illustrating color and crystal type
Photographs with satisfactory resolution can be obtained using phone cameras as well as regular digital cameras. Acceptable formats for submitted photos are jpg and png. The following considerations should be kept in mind in order to obtain the best photographs.
• Use cropped images and closeups to focus attention on the subject of interest.
• Take care with lighting to avoid reflections on glassware that obscure the contents.
• Avoid complicated and distracting backgrounds by placing blank paper or cardboard behind the subject.
For example, in the photographs below, the photo on the right provides a superior view of the reaction setup as compared to the photo on the left.
Authors may include hyperlinked videos in Organic Syntheses publications to enhance the understanding of the experimental details being described within their articles. Videos should be inserted into articles at the place in the text and Notes where they are first referred to and should be numbered and labeled as Figures with descriptive titles. At the time of submission, Authors will be asked whether their article includes videos and if that is the case, then the Associate Editor will provide instructions for uploading the video files. In the initial submitted manuscript, placeholder titles without hyperlinks should be incorporated at the appropriate position within the text.
Shorter, high-resolution movies that clearly present experimental setups and techniques not adequately captured by photographs and textual description are appropriate subjects for videos. Video files may be submitted in MPEG-4 (.MP4) or MOV (.mov) video format. It is recommended that videos should be less than 30 seconds in length and have a minimum resolution of 1080p (4K preferred). Individual file size for videos should be less than 50Mb.
Acknowledgments and Author's Contact Information
Contact information (institution where the work was carried out and mailing address for the principal author) should be included as footnotes. These footnotes should also include the email address and ORCID for the principal author. Acknowledgment of financial support should be included in footnote 1.
Biographies and Photographs of Authors
Photographs and 100-word biographies of all authors should be submitted as separate
files at the time of the submission of the procedure. The format of the biographies
should be similar to those in recent articles published on the orgsyn.org website.
Photographs can be accepted in a number of electronic formats, including tiff and
jpeg formats.
Appendix I
Determination of Purity by Quantitative Analysis
Prepared with the assistance of Dr. Margaret Faul and Dr. Christopher Borths, Process
Development, Amgen Inc. and Dr. Chris Senanayake, Chemical Development US,
Boehringer Ingelheim Pharmaceuticals, Inc.
Introduction
Common techniques for determining the purity of reaction products by quantitative NMR
("QNMR) and quantitative chromatographic analysis (HPLC and GC) are presented in this
appendix. Authors are reminded that they must provide details on equipment and conditions for GC and HPLC analyses in a Note in their article, and copies of NMR spectra and chromatograms that are used in quantitative analyses must be submitted with articles for publication.
Quantitative NMR (QNMR)1
A quick and relatively accurate method for the determination of the purity (weight percent)
of a compound can be made using proton NMR spectroscopy. A standard compound
is used for this determination. Appropriate standards are commercial compounds that
can be obtained in high (preferably >99%) purity and that produce a proton NMR signal
that is separate and distinct from the signals in the compound being analyzed. Examples
of compounds that are available in high purity and are often found useful as internal
standards include dimethyl fumarate, ethylene carbonate, and alanine.
To determine the weight percent of the target compound, 10-20 mg of the compound and an approximately equimolar amount of the standard compound (minimum of 10 mg) are accurately weighed into a clean glass container.2 The mixture of compounds is completely dissolved in approximately 1 mL of an appropriate NMR solvent such as d6-DMSO, CDCl3, etc. A proton NMR spectrum is obtained for the mixture with a minimum relaxation delay of 30 s. The weight percent purity of the analyte is calculated by the following equations:
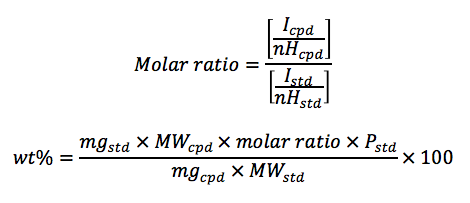
Where,
• Wt% = purity of the sample
• Icpd = proton integral area of a known peak on the compound being analyzed
• nHcpd = number of hydrogens associated with the compound NMR peak
• Istd = proton integral area of a known peak on the standard
• nHcpd = number of hydrogens associated with the standard NMR peak
• mgstd = amount in milligrams of the standard compound weighed for analysis
• mgcpd = amount in milligrams of the compound being analyzed
• MWstd = molecular weight of the standard
• MWcpd = molecular weight of the compound being analyzed
• Pstd = wt% purity of the standard expressed as a decimal value. For the purposes of most
calculations, a value of 1.00 may be assumed.
Quantitative Chromatographic Analysis
Quantitative analysis by chromatography is typically performed using either an HPLC or
GC instrument with an appropriate detector (UV-vis, FID, etc.) that has a linear response
range. Once a method is developed that cleanly resolves the peak/compound of interest,
it can easily be adapted for quantitative analysis.3 To determine purity, a response factor
for the compound of interest must be known, and a standard with known purity (e.g.,
determined by QNMR) is used to determine this.
The response factor can be calculated from a single-point standard. A single standard
sample is analyzed at a concentration within the linear dynamic range of the analytical
system and reasonably close to the expected analysis concentration. The response factor
is calculated using the following equation:
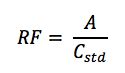
Where,
• RF = response factor
• A = area of the peak of interest
• Cstd = concentration of the standard sample4
Alternately, the response factor can be determined by generating a calibration curve by
analyzing standards at multiple concentrations. Using a multi-point calibration curve
increases the accuracy and reliability of the concentration measurement by reducing the
impact of individual weighing and measurement errors. Standard samples are prepared
so that the concentrations are within the linear dynamic range of the detector. These are
then analyzed, and the response is plotted against the concentration of each sample. The
resulting calibration curve should produce a line. The slope of this line is the response
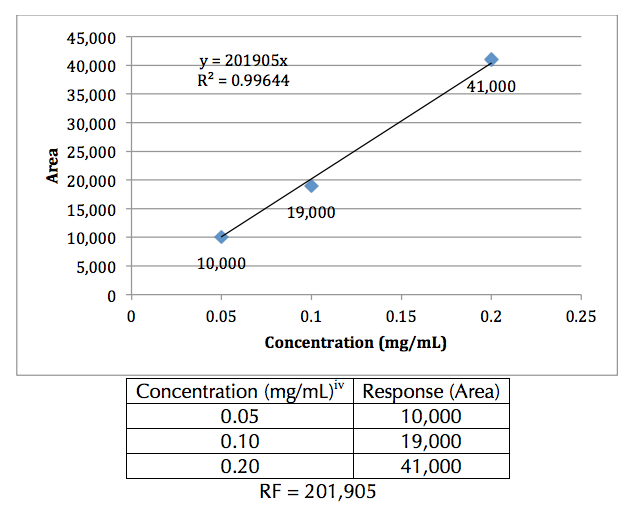
factor for the compound (Figure 1).
Figure 1. Example concentration curve. Sample (hypothetical) data values are shown in
the table. The slope of the trend line is the response factor. Note: The y-intercept was
used in the calculation of the trend line (0 mg/mL, 0 area).
Once the response factor for a compound of interest is determined, calculation of the wt%
of a sample can be done from the analysis of sample of known concentration using the
following equation:
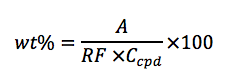
Where,
• wt% = purity of the sample
• A = area of the peak of interest
• RF = response factor for the peak of interest (previously calculated)
• Ccpd = sample concentration of the compound being analyzed
References and Notes
____________________________
1 Pinciroli, V.; Biancardi, R.; Visentin, G.; Rizzo, V. "The Well-Characterized Synthetic
Molecule: a Role for Quantitative 1H NMR", Org. Process Res. Dev. 2004, 8, 381.
2 It is more critical that >10 mg of compound and standard be used for the analysis than
that the materials be used in an equimolar ratio. Errors in mass determination are the
largest controllable source of error for this technique.
3 An acceptable chromatographic method should provide baseline separation of the peak
of interest. The peak of interest should have a response within the linear range of the
detector, have no shoulders or tailing, and should show good symmetry. This ensures that
there is no variability in the response due to overlapping analyte peaks.
4 Standard concentrations should be corrected for the purity of the standard.
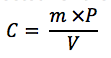
Where,
• C is the concentration of the
• m is the measured mass of the standard sample
• P is the purity factor of the standard expressed as a decimal
• V is the volume of the diluted sample