Org. Synth. 1986, 64, 85
DOI: 10.15227/orgsyn.064.0085
β-DIMETHYLAMINOMETHYLENATION: N,N-DIMETHYL-N'-p- TOLYLFORMAMIDINE
[Methanimidamide, N,N-dimethyl-N'-(4-methylphenyl)-]
Submitted by John T. Gupton and Steven A. Andrews
1.
Checked by T. V. RajanBabu and Bruce E. Smart.
1. Procedure
Caution! Cyanuric chloride is a lachrymator and causes burns on contact with the skin. All operations with this reagent should be carried out in a well-ventilated hood.
A. [3-(Dimethylamino)-2-azaprop-2-en-1-ylidene]dimethylammonium chloride. A 1-L, one-necked, round-bottomed flask is equipped with a Claisen adapter, mechanical stirrer, reflux condenser, and mineral oil bubbler (Note 1). The flask is charged with cyanuric chloride (73.8 g, 0.4 mol) (Note 2), N,N-dimethylformamide (175.4 g, 2.4 mol) (Note 3), and 1,4-dioxane (100 mL) (Note 4). The resulting solution is stirred and heated (at approximately 85°C) for 2–3 hr while a considerable amount of carbon dioxide is evolved (Note 5). When gas evolution is minimal, the reaction mixture is allowed to cool to room temperature; the product rapidly solidifies. The flask that contains the solid product is connected to an isopropyl alcohol–dry ice trap and the solvent is removed by evacuating the system to approximately 0.05 mm pressure. The crude product weighs 186–187 g (95%) and melts at 95–103°C (Note 6), (Note 7), (Note 8).
B. N,N-Dimethyl-N'-p-tolylformamidine. A 250-mL, three-necked, round-bottomed flask equipped with a reflux condenser and a magnetic stirring bar coated with Teflon is placed under a positive nitrogen pressure and charged with 100 mL of methanol (Note 9). Sodium metal (1.4 g, 0.06 mol) (Note 10) is then added in small portions. After all of the sodium has reacted, p-toluidine (6.4 g, 0.06 mol) (Note 11) is added and the resulting solution is stirred for 5 min. The iminium salt (10.6 g, 0.065 mol) produced in Step A is added in one portion and the resulting mixture is refluxed with stirring overnight. The reaction mixture is cooled to room temperature and the solvent is removed on a rotary evaporator. The residue is taken up in chloroform (100 mL) and extracted twice with a saturated, aqueous solution of sodium bicarbonate (2 × 30 mL). The chloroform phase is dried over anhydrous magnesium sulfate and filtered, and the solvent is removed on a rotary evaporator. The residual dark-brown liquid is distilled using a Kugelrohr apparatus (Note 12); the major fraction boils at 85–100°C (oven temperature), 0.4 mm, and yields 9.1–9.2 g (94–95%) of a pale-yellow liquid (Note 13) and (Note 14).
2. Notes
1.
The bubbler is connected to the condenser to monitor
carbon dioxide evolution.
2.
Cyanuric chloride was purchased from Aldrich Chemical Co., Inc. and was used without additional purification.
3.
N,N-Dimethylformamide was purchased from Aldrich Chemical Co., Inc. and was dried over Linde 3A molecular sieves prior to use.
4.
The
1,4-dioxane was reagent-grade and obtained from Fisher Scientific Corp. It was dried over Linde 3A molecular sieves prior to use.
5.
The reaction becomes very exothermic with substantial evolution of
carbon dioxide within 30–45 min after heating is initiated. It may be necessary to cool the mixture with ice water if the evolution of gas becomes too vigorous.
6.
The checkers obtained material free of
N,N-dimethylformamide after drying for at least 18 hr. The checkers found variable melting points that depended on the rate of heating. The submitters obtained
195 g (
99%) of product which melted at
81–83°C after drying overnight at 1–6 mm of pressure and indicated that the product may contain a small amount of
N,N-dimethylformamide but is suitable for use without additional purification.
[3-(Dimethylamino)-2-azaprop-2-en-1-ylidene]dimethylammonium chloride is reported to melt at
101–103°C.
27.
The product is very hygroscopic and should be handled under a moisture-free environment. If the iminium salt is kept dry, it will have a substantial shelf life. The submitters recommend storing the product in a
desiccator over anhydrous
calcium sulfate.
8.
The product has the following spectral characteristics: IR (CHCl
3) cm
−1: 1610 (C=N);
1H NMR (CDCl
3) δ: 3.27 (s, 6 H, 2 CH
3), 3.43 (s, 6 H, 2 CH
3), 9.57 (s, 2 H, -CH=N).
9.
The
methanol that was used was reagent-grade and was dried over Linde 3A molecular sieves.
10.
Sodium metal was obtained from Fisher Scientific Corp.11.
p-Toluidine was reagent-grade and was obtained from the Eastman Chemical Co.12.
The Kugelrohr apparatus was obtained from the Aldrich Chemical Co.
13.
The submitters obtained
8.3–9.1 g (
86–94%) boiling at
85–107°C, 0.4 mm. The reported boiling point of
N,N-dimethyl-N'-p-tolylformamidine is
163°C (30 mm).
3 A gas chromatographic analysis of the product using a ¼-in. × 10-ft column packed with 5% Carbowax 20 M supported on 80–100-mesh chromosorb N exhibited a single peak with a retention time of 4.8 min at an oven temperature of 220°C with a flow rate of 60 cm
3/min. The checkers redistilled the product to obtain colorless material, bp
69.5°C (0.2 mm), which was analyzed. Anal. calcd. for C
10H
14N
2: C, 74.03; H, 8.70; N, 17.27. Found C, 73.57; H, 8.51; N, 17.50
14.
The product has the following spectral characteristics: IR (neat) cm
−1: 3030 (aromatic CH), 1635 (C=N), 1600 (C=C),
1H NMR (CDCl
3) δ: 2.23 (s, 3 H, aromatic CH
3), 2.87 [s, 6 H, -N(CH
3)
2], 6.83 (d, 2 H,
J = 8, aromatic CH), 7.06 (d, 2 H,
J = 8, aromatic CH), 7.43 (s, 1 H, -CH=N-).
3. Discussion
[3-(Dimethylamino)-2-azaprop-2-en-1-ylidene]dimethylammonium chloride ("Gold's reagent"),
4 the preparation of which is described in Step A of the procedure, is a general β-dimethylaminomethylenating agent that reacts successfully with amines (Equation 1) to produce amidines,
5 with ketones (Equation 2) to produce enaminones,
6 and with amides (Equation 3) to produce acylamidines.
7 8
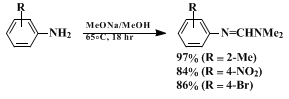
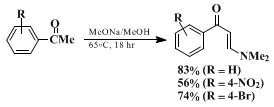
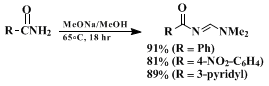
All reactions proceed in high yield and under mild conditions to produce relatively pure products. The most effective β-dimethylamino methylenating agents currently available are the formamide acetals,
9 some of which are available commercially.
10 They are, however, expensive, moisture- and heat-sensitive and require potent, mutagenic alkylating agents for their preparation. Under some circumstances they also necessitate high reaction temperatures and long reaction times. Alternatively, Gold's reagent is prepared in a single step, and in nearly quantitative yield, without purification, from inexpensive raw materials. The reaction of Gold's reagent with an amine or other substrate can be carried out at relatively low temperatures (65–90°C) and moderate reaction times (12-24 hr).
The significance of the aminomethylenated amines, ketones, and amides as important compounds and reaction intermediates is well-documented
5,6,7,8 and the use of Gold's reagent, therefore, provides an efficient, economical, and clean method
11 for obtaining such substances.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Cyanuric chloride
iminium salt
Gold's reagent
methanol (67-56-1)
chloroform (67-66-3)
sodium bicarbonate (144-55-8)
nitrogen (7727-37-9)
carbon dioxide (124-38-9)
calcium sulfate (7778-18-9)
sodium (13966-32-0)
magnesium sulfate (7487-88-9)
N,N-dimethylformamide (68-12-2)
p-toluidine (106-49-0)
1,4-dioxane (123-91-1)
[3-(Dimethylamino)-2-azaprop-2-en-1-ylidene]dimethylammonium chloride (20353-93-9)
N,N-DIMETHYL-N'-p- TOLYLFORMAMIDINE,
Methanimidamide, N,N-dimethyl-N'-(4-methylphenyl)-,
N,N-dimethyl-N'-p-tolylformamidine (7549-96-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved