Org. Synth. 1989, 67, 98
DOI: 10.15227/orgsyn.067.0098
ETHYL 5-OXO-6-METHYL-6-HEPTENOATE FROM METHACRYLOYL CHLORIDE AND ETHYL 4-IODOBUTYRATE
[6-Heptenoic acid, 6-methyl-6-oxo-, ethyl ester]
Submitted by Yoshinao Tamaru, Hirofumi Ochiai, Tatsuya Nakamura, and Zen-ichi Yoshida
1.
Checked by Kevin B. Kunnen and Albert I. Meyers.
1. Procedure
A 300-mL, four-necked, round-bottomed flask containing a magnetic stirring bar is fitted with a serum cap, a thermometer, a 100-mL serum-capped pressure-equalizing addition funnel, and a reflux condenser equipped at the top with a nitrogen inlet. The dry apparatus is flushed with nitrogen and 5.6 g (85.5 mmol) of zinc–copper couple (Note 1) and 20 mL of benzene are introduced (Note 2). A mixture of 13.8 g (57 mmol) of ethyl 4-iodobutyrate (Note 3), 9 mL of N,N-dimethylacetamide (Note 4), and 70 mL of benzene is transferred into the addition funnel by cannulation techniques and added to the stirred Zn(Cu) suspension over 3 min at room temperature. The mixture is vigorously stirred for 1 hr at room temperature (Note 5) and then heated at gentle reflux with an oil bath for 4.5 hr (Note 6). After the mixture is cooled to 60°C, a solution of 0.58 g (0.5 mmol) of tetrakis(triphenylphosphine)palladium(0) (Note 7) in 15 mL of benzene is added over 1 min through the addition funnel and stirring is continued for 5 min at the same temperature. The oil bath is removed, a solution of 5.23 g (50 mmol) of methacryloyl chloride (Note 8) in 10 mL of benzene is added through the addition funnel over a period of 5 min, and stirring is continued for 1 hr (Note 9). The mixture is filtered with suction through a Celite pad on a medium-fritted funnel and the filter cake is washed with 200 mL of diethyl ether. The filtrate is washed successively with 50 mL of 1 N ammonium chloride, 10 mL of saturated sodium hydrogen carbonate and 50 mL of saturated sodium chloride. The aqueous phases are extracted with 100 mL of diethyl ether. The combined organic extracts are dried over magnesium sulfate and the solvents are removed with a rotary evaporator to yield a deep-brown mobile oil. This is purified by chromatography on 200 g of silica gel with a hexane–diethyl ether gradient (10 : 1, 400 mL; 5 : 1, 400 mL, and 2 : 1, 600 mL) (Note 10), followed by distillation in the presence of hydroquinone (10 mg) in a Kugelrohr apparatus to give 8.0–8.1 g (87–88%) of the product as a colorless liquid, bp 185°C (20 mm) (Note 11).
2. Notes
1.
Zinc–
copper couple was prepared according to the literature procedure
2 and kept in a
desiccator over
phosphorus pentoxide under
nitrogen.
2.
Benzene is dried by distillation from
sodium/
benzophenone ketyl.
3.
Ethyl 4-iodobutyrate, bp
65°C (2.5 mm), was obtained according to the literature procedure
3 (
80–90% yield). A mixture of
50 g (0.26 mol) of ethyl 4-bromobutyrate, available from Aldrich Chemical Company, Inc., and
190 g (1.26 mol) of sodium iodide was heated in
500 mL of acetone at 60°C for 24 hr.
4.
N,N-Dimethylacetamide (DMA) is dried by distillation under reduced pressure from
calcium hydride. The use of DMA is essential to promote metallation.
4 5 The metallation is also successful with
N,N-dimethylformamide as a cosolvent, but the yield of product is significantly lowered (
60–70%) because of formation of acid anhydride.
55.
The metallation is only slightly exothermic.
6.
It is difficult to judge the completion of the metallation by appearance. Although the metallation is reproducible, it is recommended that a reaction aliquot be checked by VPC or TLC after quenching with
1 N hydrochloric acid.
7.
Tetrakis(triphenylphosphine)palladium(0) is available from Aldrich Chemical Company, Inc.8.
Methacryloyl chloride, obtained from Aldrich Chemical Company, Inc., is distilled from
calcium chloride under
nitrogen at atmospheric pressure into a flask containing a small amount of
hydroquinone monomethyl ether.
9.
The reaction is moderately exothermic, and the temperature rises to about 65°C after the addition of
methacryloyl chloride and then gradually falls to ambient temperature.
10.
The reaction mixture gives only one spot on silica gel TLC (
Rf = 0.5,
hexane/
ethyl acetate = 4 : 1 using
iodine or saturated
2,4-dinitrophenylhydrazine in 2
N hydrochloric acid as an indicator). This column purification is undertaken to help the smooth distillation of the product.
Silica gel 60 Merck in a 5.5-cm-diameter column was used.
11.
The submitters report bp
100°C (6 mm). The product shows the correct elemental analysis and has the following physical and spectral properties:
nD20 1.4512; IR (liquid film) cm
−1: 3100 (w), 2970 (m), 1730 (s), 1680 (s), 1630 (m), 940 (m);
1H NMR (CDCl
3) δ: 1.25 (t, 3 H,
J = 7.1), 1.75–2.11 (m, 5 H), 2.35 (t, 2 H,
J = 6.8), 2.76 (t, 2 H,
J = 7.1), 4.13 (q, 2 H,
J = 7.1), 5.77 (br, s, 1 H), 5.96 (s, 1 H);
13C NMR (CDCl
3) δ: 13.9, 17.2, 19.3, 33.0, 36.0, 59.9, 124.1, 144.1, 172.7, 200.6; VPC analysis:
20% Silicone DC550 on celite (Nishio Kogyo Co.), 3-mm × 1-m column, constant temperature increase 8°C/min from 100°C, one peak of impurity (retention time 1.8 min), and the peak of the product (retention time 9.0 min,
99.5% purity).
3. Discussion
α-Metallocarbonyl compounds, so-called enolates, are among the most widely used reagents for organic syntheses. Undoubtedly, β-, γ-, and δ-metallocarbonyl compounds are also of great synthetic value. The use of these organometallics, however, has been limited mainly because of the lack of a convenient preparative method. Herein are described the preparation of a γ-metallo ester,
3-carboethoxypropylzinc iodide (
1,
n = 3; Eq. 1) and its reaction with acid chlorides to yield δ-keto esters. According to the same procedure, it is possible to generate
2-carboethoxyethylzinc iodide (
1,
n = 2) and
4-carboethoxybutylzinc iodide (
1,
n = 5)
6 with similar efficiency. 2-Carboalkoxyethylzinc may be prepared by a cyclopropane ring-opening procedure (Eq 2).
7,8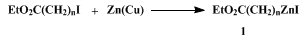
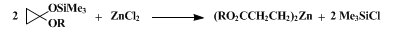
These organozincs,
1 (
n = 2, 3, and 4), react with diverse acid chlorides to yield γ-, δ-, and ε-keto esters, respectively, in good yields. Two typical examples are shown in Equations 3 and 4. The product of Equation 3,
ethyl 5-oxo-6-heptenoate, may be prepared by laborious, multistep methods.
9,10 The title compound,
ethyl 5-oxo-6-methyl-6-heptenoate, is a new compound.
Another use of
1 (
n = 3) is the coupling with
vinyl iodides or triflates, which furnish δ,ε-unsaturated esters.
11 One example is shown in Equation 5. The reaction proceeds with retention of the double-bond geometry.
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
benzophenone ketyl
vinyl iodides
6-Heptenoic acid, 6-methyl-6-oxo-, ethyl ester
calcium chloride (10043-52-4)
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
ethyl acetate (141-78-6)
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
hydroquinone (123-31-9)
sodium hydrogen carbonate (144-55-8)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
copper (7440-50-8)
iodine (7553-56-2)
acetone (67-64-1)
zinc (7440-66-6)
sodium (13966-32-0)
sodium iodide (7681-82-5)
2,4-Dinitrophenylhydrazine (119-26-6)
magnesium sulfate (7487-88-9)
N,N-dimethylformamide (68-12-2)
hexane (110-54-3)
calcium hydride (7789-78-8)
Ethyl 4-iodobutyrate (7425-53-8)
hydroquinone monomethyl ether (150-76-5)
ethyl 4-bromobutyrate (2969-81-5)
N,N-dimethylacetamide (127-19-5)
tetrakis(triphenylphosphine)palladium(0) (14221-01-3)
phosphorus pentoxide (1314-56-3)
Ethyl 5-oxo-6-methyl-6-heptenoate (130892-17-0)
METHACRYLOYL CHLORIDE (920-46-7)
3-carboethoxypropylzinc iodide
2-carboethoxyethylzinc iodide
4-carboethoxybutylzinc iodide
ethyl 5-oxo-6-heptenoate
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved