Org. Synth. 1993, 71, 236
DOI: 10.15227/orgsyn.071.0236
ETHYL 3-OXO-4-PENTENOATE (NAZAROV'S REAGENT)
[4-Pentenoic acid, 3-oxo-, ethyl ester]
Submitted by R. Zibuck
1 and J. Streiber.
Checked by Michael D. Gaul and Robert K. Boeckman, Jr..
1. Procedure
CAUTION: Acrolein is highly toxic and a lachrymator. Handle in a well-ventilated fume hood!
A. Ethyl 3-hydroxy-4-pentenoate A dry, 2-L, two-necked, round-bottomed flask, capped with septa and equipped with a thermometer (Note 1), magnetic stirring bar, and an argon inlet is flushed with argon and charged with dry tetrahydrofuran (400 mL, (Note 2)) and diisopropylamine (30.8 mL, 220 mmol, (Note 3)). The solution is cooled to −30°C and butyllithium (BuLi) (93.2 mL, 220 mmol, 2.36 M solution in hexanes, (Note 4)) is added. The reaction is stirred for 15 min and cooled to −76° to −78°C. Dry ethyl acetate (19.5 mL, 200 mmol, (Note 5)) is added dropwise so that the internal reaction temperature remains below −66°C (addition time 10–15 min). When addition of the ethyl acetate is complete, the reaction is stirred for 50 min at −70° to −78°C. A solution of freshly distilled acrolein (13.4 mL, 200 mmol, (Note 6)) and 100 mL of dry tetrahydrofuran is then added rapidly via a cannula. The reaction is stirred for 5 min and quenched by the rapid addition of saturated aqueous ammonium chloride (NH4Cl), (100 mL). The reaction mixture is poured immediately into a 2-L separatory funnel containing 500 mL of diethyl ether. The reaction flask is rinsed with 100 mL of distilled water and 100 mL of diethyl ether. After thorough mixing, the layers are separated and the aqueous layer is extracted with diethyl ether (three, 100-mL portions). The combined organic layers are washed with brine (200 mL), dried over magnesium sulfate (MgSO4), filtered, and evaporated under reduced pressure (Note 7). Crude ethyl 3-hydroxy-4-pentenoate is used in the next step (Note 8).
B. Ethyl 3-oxo-4-pentenoate. A 1-L, round-bottomed flask equipped with a magnetic stirring bar and pressure-equalizing dropping funnel is charged with ethyl 3-hydroxy-4-pentenoate (Part A) and 400 mL of acetone. The mixture is cooled in an ice bath and Jones reagent (175 mL, (Note 9)) is added dropwise via the dropping funnel (addition time is approximately 30–40 min). When addition of the Jones reagent is complete, the reaction mixture is allowed to warm slowly to room temperature and is stirred overnight (10–20 hr). Methanol (20 mL) is added to quench excess Jones reagent and the reaction mixture is poured into a 2-L separatory funnel containing diethyl ether (800 mL). After thorough mixing, the layers are separated and the aqueous layer is extracted with diethyl ether (three 200-mL portions) (Note 10). The combined organic layers are washed with brine (two 200-mL portions), dried over MgSO4, filtered, and the solvent is removed by simple distillation (Note 11). Final purification is accomplished by Kugelrohr distillation (Note 12) at 0.60 mm (oven temp 45°C) with a 250-mL receiving bulb cooled to −78°C using a dry ice/isopropyl alcohol cold bath. The purified product (14.9 g, 52%) (Note 13) can be stored at −20°C for several months without decomposition.
2. Notes
1.
A
Fluke 51 K/J digital thermometer with temperature probe is used to monitor internal reaction temperature.
2.
Tetrahydrofuran is distilled from
sodium-benzophenone ketyl under
argon. The reaction may be carried out using a freshly opened can of anhydrous
diethyl ether from Fisher Scientific or Mallinckrodt without further purification.
3.
Diisopropylamine is purified by heating to reflux over
sodium hydroxide (NaOH) for 3–12 hr, followed by simple distillation from NaOH.
4.
Butyllithium in hexanes (2.5 M) is purchased from Aldrich Chemical Company, Inc., and titrated using
diphenylacetic acid.
25.
Ethyl acetate (500 mL) is purified by washing with
100 mL of 5% sodium carbonate solution, followed by
100 mL of saturated sodium chloride solution, drying over
potassium carbonate and filtering. It is then heated at reflux over
phosphorus oxide (P2O5) for 3–12 hr and distilled from
P2O5.
36.
Acrolein is purchased from Aldrich Chemical Company, Inc. It is freshly distilled under reduced pressure (~20 mm) employing a
dry ice/isopropyl alcohol cooled-receiver.
7.
The rotary evaporator bath temperature should not exceed 40°C.
8.
If desired,
ethyl 3-hydroxy-4-pentenoate can be purified by Kugelrohr distillation at 0.60 mm (oven temperature 57°C) using a dry ice/isopropyl alcohol cold bath to cool the receiver (
79% yield). Spectral properties are as follows: IR (film) cm
−1: 3437, 2984, 2938, 1732, 1373, 1275, 1030;
1H NMR (300 MHz CDCl
3) δ 1.22 (t, 3 H, J = 6.7, OCH
2CH
3), 2.4–2.6 (m, 2 H, C(OH)CH
2C(O)), 3.15–3.25 (br m, 1 H, OH), 4.10 (q, 2 H, J = 6.7, OCH
2CH
3), 4.44–4.57 (m, 1 H, HC(OH)), 5.05–5.35 (m, 2H), 5.78–5.90 (m, 1H); MS, m/z 145 (MH
+).
9.
Jones reagent is prepared by dissolving
chromium oxide (CrO3) (23.5 g) in concd
sulfuric acid (21 mL) with cooling and then diluting with distilled water to give a total volume of 175 mL.
410.
Aqueous
chromium waste must be bottled, labeled, and given to the waste management technical personnel.
11.
The simple distillation is accomplished by using a rotary evaporator at atmospheric pressure and bath temperature ≤50°C. The last traces of solvent are removed under reduced pressure with an ambient temperature bath.
CAUTION: the product is volatile and will be lost by evaporation if care is not taken. If water should be present, the compound can be dissolved in
diethyl ether, dried again over
MgSO4, filtered and distilled.
12.
During the Kugelrohr distillation a forerun of 3–4 mL is collected and discarded.
13.
The spectral properties of
ethyl 3-oxo-4-pentenoate, which exists as a mixture of keto and enol forms, are: IR (film) cm
−1: 2984, 1741, 1659, 1588, 1423, 1242, 1150, 1038, 812;
1H NMR (CDCl
3) δ 1.2–1.3 (overlapping t, 3 H), 3.6 (s, ketonic H at C(2)), 4.1–4.3 (m, 2 H), 5.05 (s, enolic H at C(2)), 5.50 (app t, 1 H), 5.91–6.43 (m, 2 H), 11.8 (s, enol OH);
13C NMR δ 14.02, 14.19, 46.43, 60.23, 61.43, 91.84, 122.53, 130.16, 131.20; 135.74, 167.14, 168.61, 172.70, 192.62. Minor peaks are observed which may be the E enol form. The mass spectrum shows m/z 143 (MH
+).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Ethyl 3-oxo-4-pentenoate (Nazarov's reagent) is a well known annelating agent that has been used in several terpene
5 and alkaloid
6 syntheses.
Other preparations of Nazarov's reagent and its analogs have been reported,
7 but many of the procedures are labor-intensive and/or require special apparatus. The reported preparation of
ethyl 3-oxo-4-pentenoate is facile (2 steps) and efficient (
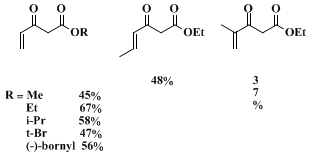
52% overall yield). All starting materials are commercially available, relatively inexpensive, and easily purified. The synthesis is also amenable to scale up and has been carried out successfully on a 1-mol scale. Other esters have also been synthesized by this method with overall yields ranging from 45–58% (see Scheme 1).
8 Finally,
methacrolein and
crotonaldehyde are also suitable reactants (see Scheme 1).
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
P2O5
brine
sodium-benzophenone ketyl
potassium carbonate (584-08-7)
sulfuric acid (7664-93-9)
ethyl acetate (141-78-6)
methanol (67-56-1)
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
sodium hydroxide (1310-73-2)
Acrolein (107-02-8)
sodium chloride (7647-14-5)
sodium carbonate (497-19-8)
acetone (67-64-1)
Diphenylacetic acid (117-34-0)
magnesium sulfate,
MgSO4 (7487-88-9)
chromium (7440-47-3)
butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
crotonaldehyde (123-73-9)
chromium oxide (1308-38-9)
methacrolein (78-85-3)
argon (7440-37-1)
diisopropylamine (108-18-9)
phosphorus oxide (1314-56-3)
ETHYL 3-OXO-4-PENTENOATE,
4-Pentenoic acid, 3-oxo-, ethyl ester (22418-80-0)
Ethyl 3-hydroxy-4-pentenoate (38996-01-9)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved