Org. Synth. 2002, 78, 14
DOI: 10.15227/orgsyn.078.0014
CATALYTIC ASYMMETRIC SYNTHESIS OF NITROALDOLS USING A LANTHANUM-LITHIUM-BINOL
COMPLEX:
(2S,3S)-2-NITRO-5-PHENYL-1,3-PENTANEDIOL
[
L-threo-Pentitol, 1,2,4-trideoxy-4-nitro-1-phenyl-
]
Submitted by Hiroaki Sasai
1a
, Shizue Watanabe, Takeyuki Suzuki, and Masakatsu Shibasaki
1b
.
Checked by Fabien Havas, Nabi A. Magomedov, and David J. Hart.
1. Procedure
A. Lanthanum-lithium-(R)-BINOL complex (LLB)
.
A dry, 300-mL, three-necked flask equipped with a magnetic
stirring bar, septum cap, and a rubber
balloon filled with argon, is charged with
(R)-2,2'-dihydroxy-1,1'-binaphthyl
((R)-BINOL; 6.49 g, 22.7 mmol
,
Note 1) and
119
mL of tetrahydrofuran
(THF, Note 2) under an argon atmosphere.
The system is placed in an ice-water bath and magnetic stirring
is initiated. Via syringe,
28.4 mL (45.4
mmol) of a 1.60 M hexane solution of butyllithium
(Note 3) is added to the cooled (R)-BINOL
solution over 7 min and the pale yellow mixture is stirred for an additional 15 min.
The cooling bath is removed and the THF solution of (R)-BINOL dilithium
salt is allowed to reach room temperature.
A 500-mL, two-necked, round-bottomed flask equipped with
a magnetic stirring bar, reflux condenser,
and a septum cap is charged with
3.12
g of lanthanum trichloride heptahydrate (LaCl3
· 7H2O, 8.4 mmol, Note 4)
and
100 mL of THF. The resulting
suspension is sonicated for 30 min at room temperature (Note 5).
To this suspension is added the above prepared solution of
(R)-BINOL
dilithium salt
via syringe over 5 min with vigorous stirring
(Note 6). After this mixture is stirred for 30 min at room temperature,
a 0.52 M THF solution of sodium
tert-butoxide (4.85 mL, 2.52 mmol, Note
7) is added via syringe over 5
min. The resulting suspension is stirred vigorously for 14 hr at room temperature
and then stirred for 48 hr at 50°C. The reaction mixture is allowed to cool to room
temperature without stirring, and the supernatant is used as a 0.03
M solution of lanthanum-lithium-(R)-BINOL catalyst [(R)-LLB].
B. (2S,3S)-2-Nitro-5-phenyl-1,3-pentanediol
.
A 500-mL, two-necked, round-bottomed flask equipped with a
magnetic stirring bar, septum cap, and
a rubber balloon filled with argon, is charged with 119 mL of THF and a 0.03 M THF
solution of (R)-LLB catalyst (63.3 mL, 1.90 mmol)
under an argon atmosphere. The system is cooled to −40°C
and magnetic stirring is initiated (Note 8). The mixture is stirred
for 30 min at −40°C, then
2-nitroethanol
(3.00 mL, 41.8 mmol, Note 9) is added via syringe over 4 min. After 30 min of stirring
at −40°C,
5.00 mL of 3-phenylpropanal
(38.0 mmol, Note 10)
is added via syringe over 5 min and the resulting solution is stirred for 90 hr. The
reaction is monitored by TLC (Note 11). To the reaction mixture
is added
150 mL of 1N hydrochloric
acid (HCl). To this mixture is added
20
g of sodium chloride (NaCl) and the resulting
mixture is transferred to a 2-L separatory funnel. The aqueous
phase is extracted three times with
ethyl acetate
(400, 200, and 200 mL) and the combined organic phases
are washed with
350 mL of aqueous saturated
NaCl solution and dried over
sodium
sulfate
. The solvent is removed with a rotary evaporator,
and the resulting crude product is recrystallized from 1
: 1 hexane: ether (ca. 200 mL)
to give 4.31g (50%) of
analytically
pure
(2S,3S)-2-nitro-5-phenyl-1,3-pentanediol
(98% ee, Notes 12-15).
2. Notes
1.
(R)-BINOL was dried at 50°C for 2 hr under
reduced pressure.
2.
THF was freshly distilled from
sodium
benzophenone ketyl under an
argon atmosphere.
3.
BuLi was purchased
from Kanto Chemical Company, Inc.
and titrated prior
to use.
4.
Pulverized
lanthanum chloride
heptahydrate was purchased from Aldrich Chemical Company, Inc.
(purity 99.9%), or Kanto Chemical Company, Inc.
(purity 99.99%). The anhydrous salt is very hygroscopic
and must be protected against moisture. It can, however, be handled very quickly in
air without any special precaution.
5.
The checkers used a
Branson sonicator.
6.
The transfer may also be made via
cannula.
7.
Sodium tert-butoxide
was purchased from Aldrich Chemical Company, Inc.
8.
When the checkers conducted the reaction at −30°C, a 45%
yield of product with 50% ee was obtained.
9.
2-Nitroethanol was distilled under reduced
pressure prior to use.
10.
3-Phenylpropanal was distilled under reduced
pressure prior to use.
11.
Acetone
-
hexane (1:2) or
dichloromethane-methanol
(20 : 1) is used as an eluant.
Anisaldehyde
is used as an indicator. Silica gel is used as the stationary phase.
12.
The submitters indicate that purification of a small amount of
crude
2-nitro-5-phenyl-1,3-pentanediol by reverse phase chromatography
(
Lobar LiChroprep RP-8, CH
3CN-H
2O 1:1)
revealed that the yield, diastereoselectivity, and optical purity of the syn adduct
is 79%, syn: anti = 11.5 : 1, and 89% ee, respectively.
13.
The submitters obtained a second crop:
0.60 g (
7%
yield, 91% e.e.).
14.
The enantiomeric excess was determined by HPLC analysis using DAICEL CHIRALPAK AD (
hexane:
i-PrOH 9:1, 0.7 mL/min, 254 nm). The retention times for the
(2R,3R)-, (2S,3S)-, (2R,3S)- and (2S,3R)-derivatives are 22.6 min, 24 min, 17 min
and 18 min, respectively. The analytical and spectral data of pure
(2S,3S)-2-nitro-5-phenyl-1,3-pentanediol
are as follows:
[α]D
24
−17.7° (c 0.86, chloroform, 98% ee),
mp
101-102°C;
1H NMR δ: 1.83-1.95 (m, 2 H), 2.19
(br-t, 1 H, J = 6.2), 2.44 (br-d, 1 H, J = 7.5), 2.75 (m,
1 H), 2.90 (m, 1 H), 4.03-4.23 (m, 3 H), 4.57
(ddd, 1 H, J = 4.0, 5.2, 6.7), 7.17-7.35 (m, 5 H)
;
13C NMR δ: 31.45 (CH),
35.15 (CH
2), 61.78 (CH
2), 69.53
(CH
2), 92.08 (CH), 126.33 (CH), 128.43
(CH), 128.64 (CH)
140.56 (C)
; IR (KBr) cm
−1: 3332, 1559,
1369
; FABMS (glycerol)
m/z 226 (M
++1), 161, 91
(base peak).
Anal. calcd for C
11H
15NO
4: C, 58.66; H, 6.71; N,
6.22. Found: C, 58.47; H, 6.77; N, 5.93. The checkers obtained a
53% yield of product, pure by combustion analysis, with
95% ee. One recrystallization of this material (85% recovery) gave product with greater
than 98% ee. When the checkers used catalyst aged for 16 days at −20°C,
product was obtained in
43%
yield with a 95% ee.
15.
The submitters recovered BINOL in quantiative yield without racemization.
This material was reused after recrystallization from
toluene.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The
lanthanum-lithium-BINOL (LLB) catalyst
2
can be prepared by three different methods that start with
lanthanum trichloride,
3
lanthanum
trichloride heptahydrate (procedure A),
4 or lanthanum tris(2-propoxide).
5,6 From a practical perspective,
the most important method is procedure A, since this procedure is easy to perform,
and the starting
lanthanum trichloride heptahydrate is much cheaper
than the corresponding anhydride or
lanthanum tris(2-propoxide).
Using methods similar to those for LLB, other rare earth-lithium-BINOL complexes can
be prepared. For example,
praseodymium-lithium-BINOL complex
(PrLB) and
neodymium-lithium-BINOL complex (NdLB) can be prepared
from
praseodymium tris(2-propoxide) and
neodymium tris(2-propoxide),
respectively. In some cases, these rare earth-lithium-BINOL complexes promote the
nitroaldol reaction more efficiently than LLB to give nitroaldols in higher optical
purities.
6,7 Furthermore,
samarium-lithium-BINOL
complex (SmLB),
europium-lithium-BINOL complex (EuLB)
and
gadolinium-lithium-BINOL complex (GdLB) are effective in
the asymmetric nitroaldol reaction of aromatic aldehydes with
nitromethane.
6,7 The purity of rare earth tris(2-propoxide)
may vary with supplier. The submitters have obtained their best results using rare
earth tris(2-propoxide) purchased from Kojundo Chemical Laboratory Co., Ltd., Japan.
Concerning the source of the chiral catalysts, the introduction of trialkylsilylethynyl
groups at the 6,6'-position of BINOL is effective for obtaining nitroaldols
in higher enantiomeric excesses.
8 In the case of diastereo- and enantioselective
nitroaldol reactions using
nitroethane,
nitropropane,
and
2-nitroethanol, higher diastereomeric excesses are also observed
using
6,6'-bis(triethylsilylethynyl)BINOL as a ligand.
8 Furthermore, the submitters have synthesized several therapeutically
or biologically important compounds such as β-blockers,
7,9,10,11 norstatine derivative,
12 and threo-dihydrosphingosine.
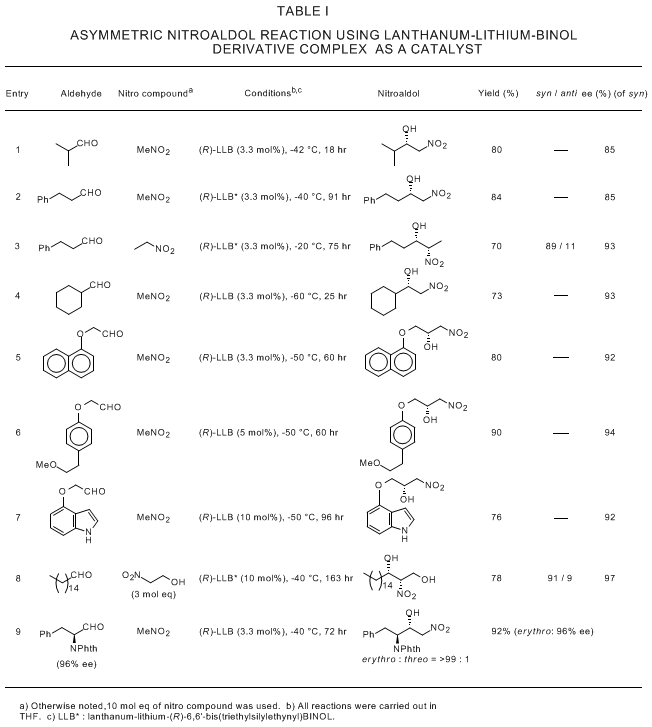
8 Representative results are shown in Table I.
To the best of the submitter's knowledge, rare earth-lithium-BINOL complexes are the
only catalysts that efficiently promote asymmetric nitroaldol reactions.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(2S,3S)-2-Nitro-5-phenyl-1,3-pentanediol:
L-threo-Pentitol,
1,2,4-trideoxy-4-nitro-1-phenyl- (9); (172171-05-0)
(R)-2,2'-Dihydroxy-1,1'-binaphthyl
[
(R)-BINOL]:
[1,1'-Binaphthalene]-2,2'-diol,
(R)- (+)- (8);
[1,1'-Binaphthalene]-2,2'-diol, (R)-
(9); (18531-94-7)
Butyllithium:
Lithium, butyl-
(8,9); (109-72-8)
Lanthanum trichloride heptahydrate:
Aldrich:
Lanthanum chloride heptahydrate (8);
Lanthanum
chloride, heptahydrate (9); (10025-84-0)
Sodium tert-butoxide:
tert-Butyl
alcohol, sodium salt (8);
2-Propanol, 2-methyl-, sodium salt
(9); (865-48-5)
2-Nitroethanol:
Ethanol,
2-nitro- (8,9); (625-48-9)
3-Phenylpropanal: Aldrich:
Hydrocinnamaldehyde
(8);
Benzenepropanal (9); (104-53-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved