Org. Synth. 2002, 79, 139
DOI: 10.15227/orgsyn.079.0139
CATALYTIC ENANTIOSELECTIVE ADDITION OF DIALKYLZINCS TO ALDEHYDES USING (2S)-(−)-3-exo-(DIMETHYLAMINO)ISOBORNEOL
[(2S)-DAIB]: (S)-1-PHENYL-1-PROPANOL
[
Benzenemethanol, α-ethyl-, (S)-
]
Submitted by Masato Kitamura, Hiromasa Oka, Seiji Suga, and Ryoji Noyori
1
.
Checked by David E. Kaelin, Stephen F. Martin, Gregory L. Beutner, and Scott E. Denmark.
1. Procedure
Caution! Since DAIB deteriorates in air, bottles of DAIB should be flushed
with N2 or Ar and kept tightly closed for storage over long periods. Diethylzinc
easily catches fire upon contact with air or moisture and addition to benzaldehyde
should be performed under anaerobic conditions using degassed solvent.
A dry, 500-mL Schlenk tube, equipped with a rubber
septum and a Teflon-coated stirring bar and filled
with argon
(Note 1) is charged with
(2S)-(−)-3-exo-(dimethylamino)isoborneol
[(2S)-DAIB]
(371 mg, 1.88 mmol)
(Note 2),
dry toluene (200 mL)
(Note 3), and a 4.45 M toluene
solution of
diethylzinc
(25.4
mL, 113 mmol) through a rubber septum
using hypodermic syringes at 20°C (Notes 4 and 5). The mixture is stirred
for 15 min and then cooled to −78°C with a dry ice-methanol bath.
To this is added
benzaldehyde (10.0
g, 94.2 mol)
(Note 6) in one
portion (Note 7). The bath is replaced by an ice bath,
and the septum is replaced by a glass stopper. The reaction
mixture is stirred at 0°C for 6 hr in a closed system. The glass stopper
is removed under an argon stream, and saturated
aqueous ammonium chloride solution (40 mL)
is carefully added (Note 5), resulting in the formation of a
white precipitate. The liquid layer and the solid phase are roughly separated by decantation.
The precipitate is washed with ether (100 mL),
and the combined liquid layers and 2 M aqueous hydrochloric
acid solution (100 mL) are transferred to a
1-L separatory funnel. The aqueous layer is separated and extracted
twice with ether (100 mL)
(Note 8). The combined organic layers (ca. 550 mL) are washed with water
(50 mL) and brine (50 mL), dried
over anhydrous sodium sulfate
,
and concentrated under reduced pressure. The crude residue is distilled at 150-155°C and 20 mm Hg using a Kugelrohr
apparatus to give 12.4 g
of
(S)-1-phenyl-1-propanol
(97% yield) in 95.4% ee as
a colorless oil (Notes 9 and 10).
2. Notes
1.
Argon gas (99.998%) is used without further
purification. The
Schlenk tube and
syringes
are dried overnight at 150°C.
2.
Supplied by Professor James D.White Oregon State University;
2 see previous procedure, p.130.
3.
Toluene
is first distilled from
sodium benzophenone ketyl
under argon. Prior to the reaction, the
toluene
is degassed by performing two freeze-pump-thaw cycles, then the flask is backfilled
with
argon.
4.
A stock solution of
diethylzinc
is prepared in an
80-mL Schlenk tube equipped with a
Young's
tap by mixing
toluene
and
99%
diethylzinc
[(16.5 g, 134 mmol) in a lecture bottle,
which is purchased from Aldrich Chemical Company, Inc.]
to make a total volume of 30 mL.
5.
Ethane
gas evolution is observed.
6.
Benzaldehyde, which
is purchased from Aldrich Chemical Company, Inc., is purified
by distillation from 4 Å molecular sieves
(70.5-71.5°C/20
mmHg) and kept in a
Schlenk tube equipped with a
Young's
tap.
7.
The solution becomes a pale yellow color; the color fades after
6 hr.
8.
(2S)-DAIB (312 mg)
is recovered in 77–84% yield from the aqueous layer. To the aqueous layer (pH
<2), cooled with an
ice bath, is added
6
M aqueous sodium hydroxide solution (60 mL).
The mixture, which has a pH of >12, is extracted three times with
ether
(50 mL). The combined organic layers are washed with water
(20 mL) and
brine (20 mL), dried
over
anhydrous sodium sulfate
,
and concentrated under reduced pressure to give a colorless oil.
9.
The product, which contains
2-3%
of
benzyl alcohol
and
2-3% of
propiophenone
, has the following
physical properties:
[α]D
21
−45.6° CHCl3, c 5.55);
1H NMR (400 MHz, CDCl
3) δ:
0.91 (t, 3, J = 7.3, CH
3), 1.68-1.88 (m, 2, CH
2),
1.90-1.95 (m, 1, OH), 4.58 (td, 1, J = 6.4, 3.4, OCH), 7.21-7.38
(5, aromatic protons)
;
13C NMR (100 MHz, CDCl
3) δ: 10.1,
31.9, 76.0, 125.9, 127.5, 128.4,
144.6
.
10.
The enantiomeric purity is determined by chiral stationary phase,
supercritical fluid chromatographic (CSP-SFC) analysis (Berger Instruments, Daicel
Co. CHIRALCEL OD column;
4%
methanol
,
180 psi, 3.0 mL/min flow rate; detection at 220 nm).
Racemic
1-phenylpropanol
exhibited base-line separation of
peaks of equal intensity arising from the R-isomer (t
R 2.74 min) and the
S-isomer (t
R 3.10 min) whereas the synthetic alcohol showed these peaks
in the ratio 97.7 / 2.3. This chromatographic method allowed for identification of
the trace contaminants
propiophenone
(t
R 1.63 min) and
benzyl alcohol
(t
R 3.40 min).
11.
The submitters used HPLC analysis to determine the enantiomeric
purity (Daicel Co. CHIRALCEL OB column; 99.5/0.5
hexane/2-propanol
mixture, 1.0 mL/min flow rate; detection at 254-nmS-isomer t
R 19.2 min,
R-isomer t
R 24.6 min)). Under these conditions, however, the propiophenone
contaminant is coincident with the S-enantiomer thus affording unreliable enantiomeric
analysis.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
(2S)-DAIB, a chiral β-dialkylamino alcohol, serves as an efficient catalyst
for enantioselective addition of
diethylzinc
to
benzaldehyde
in
toluene
,
hexane
,
ether
, or their mixtures, giving
(S)-1-phenyl-1-propanol
in up to 99% ee.
3
4 This catalytic enantioselective
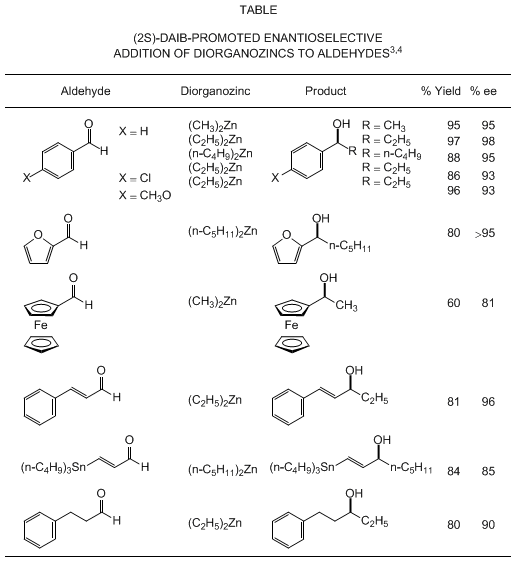
alkylation can be extended to a range of alkylating agents and aldehyde substrates,
as illustrated in the Table. Dimethyl-, diethyl-, and other simple dialkylzinc agents
can be used as alkylating agents. p-Substituted benzaldehydes give the corresponding
secondary alcohols with consistently high enantioselectivity.
2-Furaldehyde
is alkylated with
di-n-pentylzinc
in the presence of (2S)-DAIB to give
(1S)-1-(2-furyl)hexan-1-ol
,
a versatile compound in organic synthesis, with >95% ee. Optically active
1-ferrocenylethanol
, a key
compound for the synthesis of a wide variety of chiral ferrocene derivatives, is obtained
in
81% optical yield by methylation
of
ferrocenecarboxaldehyde
.
Certain α,β-unsaturated and aliphatic aldehydes can also be alkylated in
moderate to high optical yield. The (2S)-DAIB-catalyzed addition of
di-n-pentylzinc
to
(E)-3-tributylstannylpropenal
proceeded with an S:R selectivity of 93:7 to afford a key chiral building block that
was used in the three-component coupling step of a prostaglandin synthesis. The DAIB-catalyzed
reaction of (1-alkenyl)ethylzincs, prepared by transmetalation of (1-alkenyl)dicyclohexylboranes
with
diethylzinc
, plays
a key role in the asymmetric syntheses of muscone and aspicilin.
5 Polystyrene-anchored DAIB can
also be used as a chiral auxiliary for enantioselective reactions.
6
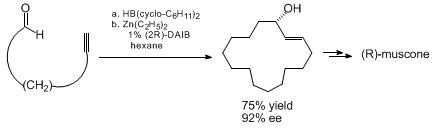
Dramatic chiral amplification is observed in alkylations catalyzed by partially
resolved DAIB.
7 Reaction of
benzaldehyde
and
diethylzinc
in
toluene
containing
8
mol% of (2S)-DAIB of 15% ee leads to
(S)-1-phenyl-1-propanol
in 95% ee, a value close to the 98% ee obtained with enantiomerically pure (2S)-DAIB.
A combined system consisting of a Ni(II) complex and (2S)-DAIB effects the conjugate
addition of
diethylzinc
to
chalcone
, resulting in
the formation of
(R)-1,3-diphenylpentan-1-one
in 85% ee.
8
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(2S)-3-exo-Aminoisoborneol:
Bicyclo[2.2.1]heptan-2-ol,
3-amino-1,7,7-trimethyl-, [1R-(exo,exo)]; (41719-73-7)
(2S)-3-exo-(Dimethylamino)isoborneol:
Bicyclo[2.2.1]heptan-2-ol,
3-(dimethylamino)-
1,7,7-trimethyl-, [1R-(exo,exo)]; (103729-96-0)
(S)-1-Phenyl-1-propanol:
Benzenemethanol,
.alpha.-ethyl-, (S)-; (613-87-6)
(R)-1-Phenyl-1-propanol:
Benzenemethanol,
.alpha.-ethyl-, (R)-; (1565-74-8)
(±)-1-Phenyl-1-propanol:
Benzenemethanol,
.alpha.-ethyl-, (93-54-9)
Benzaldehyde:
Benzaldehyde; (100-52-7)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved