Org. Synth. 1928, 8, 26
DOI: 10.15227/orgsyn.008.0026
BENZOPHENONE
Submitted by C. S. Marvel and W. M. Sperry.
Checked by J. B. Conant and G. M. Bramann.
1. Procedure
In a 5-l. two-necked, round-bottomed flask fitted with a good mechanical stirrer (Note 1), a separatory funnel, a thermometer, and a reflux condenser connected with a trap (Note 2) for absorbing the hydrogen chloride evolved, are placed 455 g. (3.4 moles) of anhydrous aluminum chloride (Note 3) and 1 l. (10.2 moles) of dry carbon tetrachloride (Note 4). The flask is surrounded by an ice bath (Note 5). The stirrer is started and when the temperature of the carbon tetrachloride has dropped to 10–15°, 50 cc. of dry thiophene-free benzene (Note 6) is added all at once. The reaction begins immediately as is indicated by the evolution of hydrogen chloride and a rising temperature. As soon as the reaction has started, salt is added to the ice in the cooling bath in order to get more effective cooling. When the temperature begins to fall after the reaction has started, a mixture of 550 cc. (a total of 6.7 moles) of thiophene-free benzene and 550 cc. (a total of 14.5 moles) of carbon tetrachloride is run in at such a rate that the temperature is kept between 5° and 10° (Note 5).
If efficient cooling is maintained, this addition requires one to two hours. The stirring is continued for about three hours after the benzene-carbon tetrachloride solution has been added, while the temperature is held at about 10°. The stirring is then discontinued and the mixture is allowed to stand about twelve hours. During this time the mixture comes to room temperature.
The stirrer is then again started and about 500 cc. of water is slowly added. External cooling is used in order that the water may be added more rapidly. The excess
carbon tetrachloride usually refluxes during this part of the procedure. The reaction mixture is then first heated on a
steam bath to remove most of the excess of
carbon tetrachloride, then the mixture is distilled with steam to carry over the remaining
carbon tetrachloride (Note 7), and to hydrolyze the
benzophenone dichloride to
benzophenone. The
carbon tetrachloride comes over in about thirty minutes but the steam distillation is continued for about one hour to insure complete hydrolysis. The upper
benzophenone layer is then separated from the aqueous layer and the latter is extracted with about
200 cc. of benzene. The
benzene solution and the
benzophenone are transferred to a
1-l. modified Claisen flask (p. 130) for distillation. The
benzene and any water that is present are removed under ordinary pressure and the
benzophenone is distilled under reduced pressure
(Note 8).
The yield is
490–550 g. (
80–89 per cent of the theoretical amount based on the
benzene) of a product boiling at
187–190° /15 mm., and solidifying to a white solid melting at
47–48°. The material sometimes has a bluish tinge. This color may be removed and a colorless product obtained by moistening the material with
benzene and centrifuging.
2. Notes
1.
The stirrer should be very efficient, as otherwise the
aluminum chloride tends to cake on the sides of the flask. This makes cooling very difficult and thus increases the time necessary for the addition of the
benzene-carbon tetrachloride mixture.
2.
Gas Absorption Trap.—A convenient trap (Fig. 7) devised by John R. Johnson for the absorption of
hydrogen chloride, or for the elimination of
sulfur dioxide,
hydrogen cyanide, etc., may be arranged as shown in the figure.
Fig. 7.
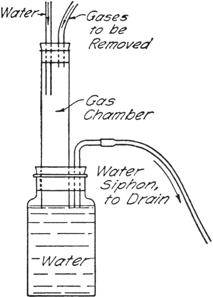 |
The gases are led into a chamber in which a stream of water (from the reflux condenser in this case) flows downward into a large bottle. The bottle is provided with a bent tube which serves as a siphon drain. The gases are thus brought into contact with a flowing stream of water so that the heat of solution is dissipated, and the level of the water in the lower bottle serves as a seal to prevent escape of the gases into the atmosphere. Water-insoluble gases are drawn out through the siphon drain directly into the sink.
If the gas chamber is of sufficient capacity there is practically no danger of water being drawn back into the reaction vessel, but care should be exercised when the reaction flask is cooled. For the reaction described here, a gas chamber about 2.5 cm. in diameter and 20–25 cm. in length was found to be satisfactory.
An alternative, and simpler, gas absorption trap is described in Org. Syn. 14, 2.
In reactions involving compounds sensitive to water it is desirable to introduce a drying tube between the condenser and the trap in order to absorb any moisture which might diffuse up from the trap.
3.
A good grade of technical anhydrous
aluminum chloride was used to obtain the results given in the procedure. The yield decreases considerably when the quality of this reagent is not good.
4.
No difference in yield is noticed in using the ordinary "pure" grade of
carbon tetrachloride and the
sulfur-free c.p. grade. It is easily dried by distilling the commercial product and rejecting the first 10 per cent of the distillate.
5.
It is necessary to allow the reaction to start before packing in an ice-salt mixture. If the temperature is too low (below 10°) the reaction does not start. After the reaction has started, the cooling should be as efficient as possible so that the mixture of
benzene and
carbon tetrachloride may be added in the minimum amount of time. If the temperature drops below 5° the reaction is too slow. If the temperature goes above 10° there is increasing formation of tarry matter and lowering of the yield.
6.
The yield is 5 to 10 per cent lower if the ordinary technical grade of
benzene is used. The
benzene is dried in the same manner as the
carbon tetrachloride (Note 4).
7.
About
1050–1150 cc. of carbon tetrachloride is recovered. This contains a small amount of
benzene. However, it may be used in a succeeding run if it is dried over
calcium chloride and distilled.
No difference in the yield is noticed when recovered
carbon tetrachloride is used.
8.
There is considerable tendency for the
benzophenone to foam over during the early part of the distillation under reduced pressure and care must be taken to prevent this.
3. Discussion
Benzophenone can be prepared by the distillation of
calcium benzoate,
1 by the action of
benzoyl chloride on
benzene in the presence of
aluminum chloride,
2 by the
action of
phosgene on
benzene in the presence of
aluminum chloride,
3 by the action of
carbon tetrachloride on
benzene in the presence of
aluminum chloride followed by hydrolysis,
4 by treating
benzoic anhydride in
benzene and
acetic anhydride with
aluminum chloride,
5 by heating
o-benzoyl-benzoic acid with a small amount of its copper salt,
6 by oxidizing
diphenylmethane with
nitric acid in the presence of
lead acetate,
7 and by adding
phenylmagnesium chloride to
benzoyl chloride.
8
This preparation is referenced from:
- Org. Syn. Coll. Vol. 1, 8
- Org. Syn. Coll. Vol. 1, 46
- Org. Syn. Coll. Vol. 1, 84
- Org. Syn. Coll. Vol. 1, 109
- Org. Syn. Coll. Vol. 1, 121
- Org. Syn. Coll. Vol. 1, 142
- Org. Syn. Coll. Vol. 1, 147
- Org. Syn. Coll. Vol. 1, 166
- Org. Syn. Coll. Vol. 1, 241
- Org. Syn. Coll. Vol. 1, 256
- Org. Syn. Coll. Vol. 1, 292
- Org. Syn. Coll. Vol. 1, 323
- Org. Syn. Coll. Vol. 1, 341
- Org. Syn. Coll. Vol. 1, 394
- Org. Syn. Coll. Vol. 1, 473
- Org. Syn. Coll. Vol. 1, 495
- Org. Syn. Coll. Vol. 1, 506
- Org. Syn. Coll. Vol. 1, 517
- Org. Syn. Coll. Vol. 1, 533
- Org. Syn. Coll. Vol. 1, 544
- Org. Syn. Coll. Vol. 1, 548
- Org. Syn. Coll. Vol. 2, 3
- Org. Syn. Coll. Vol. 2, 70
- Org. Syn. Coll. Vol. 2, 71
- Org. Syn. Coll. Vol. 2, 606
- Org. Syn. Coll. Vol. 3, 547
- Org. Syn. Coll. Vol. 4, 178
- Org. Syn. Coll. Vol. 6, 520
- Org. Syn. Coll. Vol. 9, 151
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
calcium chloride (10043-52-4)
hydrogen chloride (7647-01-0)
Benzene (71-43-2)
acetic anhydride (108-24-7)
hydrogen (1333-74-0)
nitric acid (7697-37-2)
sulfur dioxide (7446-09-5)
carbon tetrachloride (56-23-5)
cyanide (57-12-5)
sulfur (7704-34-9)
benzoyl chloride (98-88-4)
Benzoic anhydride (93-97-0)
aluminum chloride (3495-54-3)
Benzophenone (119-61-9)
benzene-carbon tetrachloride
benzophenone dichloride
calcium benzoate (2090-05-3)
phosgene (75-44-5)
Diphenylmethane (101-81-5)
lead acetate
phenylmagnesium chloride (100-59-4)
o-benzoyl-benzoic acid (85-52-9)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved