Org. Synth. 1923, 3, 47
DOI: 10.15227/orgsyn.003.0047
EPICHLOROHYDRIN
Submitted by H. T. Clarke and W. W. Hartman.
Checked by Roger Adams and H. O. Calvery.
1. Procedure
In a
5-l. flask (Note 1) provided with a
mechanical stirrer, a
reflux condenser, and a
hopper which can be opened or closed at the bottom by means of a
rubber bung attached to a
glass rod (Fig. 12),
Fig. 12.
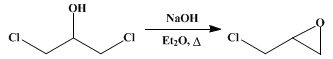 |
are placed
3 l. of anhydrous ether and
1290 g. (943 cc., 10 moles) of glycerol α,γ-dichlorohydrin (p. 292). The flask is surrounded by a
cold-water bath, and
440 g. (11 moles) of finely powdered sodium hydroxide (Note 2), which has been passed through a
20-mesh sieve, is added through the hopper in small portions, with continual stirring, while the temperature is kept at 25–30°. The addition requires about twenty minutes. The cold water is replaced by water at 40–45°, and the mixture boiled gently with stirring for four hours. It is necessary to cool the
vessel at least once every hour during this period and break up with a rod or
wire any lumps which cling to the side of the flask and are not incorporated by the stirrer.
The mixture is finally cooled and the ethereal solution carefully decanted from the solid, which is carefully rinsed twice with 250-cc. portions of dry ether. The united liquids are then distilled from a water bath held at 46–60°, the residue is fractionated with a column, and the fractions boiling at the following points are collected: up to 110°; at 110–115°; at 115–117°; and at 117–140°. The portion boiling at 115–117° is pure epichlorohydrin; the lower and higher fractions are systematically redistilled, yielding a further quantity of pure material. The yield is 705–747 g. (76–81 per cent of the theoretical amount). The residue, varying from 16 to 150 g., consists of nearly pure glycerol dichlorohydrin, and may be employed in subsequent runs (Note 3).
2. Notes
1.
A
three-necked flask is very satisfactory for this reaction; if it is not available, the tubes leading from the reflux condenser and the hopper must be bent at slight angles to prevent congestion of apparatus.
2.
The principal difficulties in the preparation are connected with the use of finely powdered alkali. Care must be taken to expose the powder as little as possible to a moist atmosphere, for if it becomes at all damp it tends to clump together and difficulty is experienced in adding it to the mixture. For this reason, and also on account of the irritating action of alkali on the mucous membranes, the sieve should be provided with well-fitting cover and receiver; the sifted material should be weighed out into a
stoppered bottle and placed in the apparatus directly from this container. The hopper (Fig. 12) from which the alkali is added to the mixture should be covered with a card with a hole through which the rod passes. The bung on the rod may conveniently be constructed from a
rubber stopper of appropriate size.
3.
It is not essential to redistil the recovered dichlorohydrin, since the
glycerol which forms the principal by-product is retained by the excess alkali and does not enter the
ether.
3. Discussion
Epichlorohydrin can be prepared by the action of alkalies on
glycerol α- and β-dichlorohydrins.
1 An alternative procedure involving the use of
calcium hydroxide has also been described.
2
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Glycerol α,γ-dichlorohydrin
glycerol dichlorohydrin
glycerol α- and β-dichlorohydrins
ether (60-29-7)
sodium hydroxide (1310-73-2)
glycerol (56-81-5)
Epichlorohydrin (106-89-8)
calcium hydroxide
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved