Org. Synth. 1921, 1, 49
DOI: 10.15227/orgsyn.001.0049
FURFURAL
[2-Furaldehyde]
Submitted by Roger Adams and V. Voorhees.
Checked by H. T. Clarke and E. R. Taylor.
1. Procedure
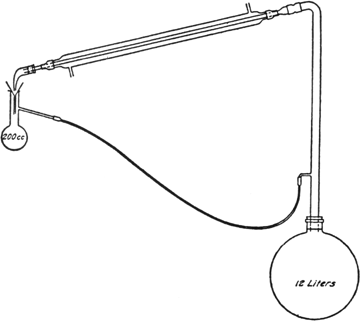
In a
12-l. round-bottomed flask are placed 1.5 kg. of dry corn cobs (ground to about the size of corn kernels)
(Note 1),
5 l. of 10 per cent sulfuric acid, and 2 kg. of salt. The flask is shaken in order to secure a homogeneous mixture and is then connected with an
upright tube,
water condenser, and
return tube as shown in
Fig. 17, p. 282 (Note 2). Heat is applied from a
ring burner, the flame being adjusted so that the liquid distils at a rapid rate.
The distillation process is continued until practically no more furfural can be seen collecting in the distilling flask used as a receiver. The above operation requires from five to ten hours.
This distillate is now treated with enough sodium hydroxide so that the mixture is left just faintly acid, and the furfural separated. It amounts to 180–220 g. The wet furfural is distilled under reduced pressure from a Claisen flask which is heated in an oil bath (Note 3). The temperature of the bath is never permitted to rise above 130°. At first, water together with some furfural distils, and this fraction is separated to be worked up with a later portion. Finally, 165–200 g. of pure furfural (b.p. 90°/65 mm.; 159°/745 mm.) distils, and this fraction, collected separately, is found to be practically colorless. The distillation of the crude material must not be carried out under ordinary pressure; otherwise the product turns dark rapidly on standing. After one distillation under reduced pressure as described, however, a distillation under atmospheric pressure may be carried out without the objectionable results just mentioned (Note 4).
2. Notes
1.
Certain samples of ground cobs which had remained in the laboratory for a year did not give nearly such good yields of
furfural as fresher ones.
2.
The apparatus described (Fig. 17) is very convenient for laboratory use, but may be modified in many ways as long as a few essential points are kept in mind. A modification worth mentioning is the use as a receiver of a vessel the bottom of which has a
stopcock attached, thus allowing the
furfural to be drawn off at any time. It is necessary to have the receiver several inches higher than the opening in the upright tube so that the aqueous liquors will flow back to the reaction flask; the lower end of the funnel in the receiver must be below the side arm of the receiver in order to prevent bubbles of
furfural from collecting on the surface of the liquid and being carried back to the reaction flask; the entrance of the returning liquid into the upright tube must be at such a point that a vigorous stream of vapor passes through the returning liquid, thus extracting much of the
furfural carried back in the water; the tube for returning the aqueous liquors must at some point be lower than the entrance to the upright tube so that a trap of liquid will be formed and prevent the vapors from the reaction flask entering the side tube. If the return tube is small and swings too low, it happens occasionally that a bubble of air gets into the tube and prevents the regular flow of liquid; this can be remedied by shaking the tube until the difficulty is overcome. It was found that the more efficient the upright tube was as a
fractionating column, the more complete was the separation of
furfural.
Fig. 17.
3.
Distillation of the product under reduced pressure is essential. Moreover, in this final distillation the precautions mentioned (the use of an oil bath and an outside temperature of less than 130°) must be carefully observed. When the
furfural is distilled under ordinary pressure or when it is distilled under reduced pressure with a free flame, a practically colorless product is at first obtained. After a few days and sometimes after a few hours this product will gradually darken until finally a black liquid results. This change, although most marked in the presence of light, takes place readily even when the
aldehyde is stored in
brown glass bottles. On the other hand, when the crude
furfural is distilled under reduced pressure and is never heated above 130° during the process of distillation, a product is obtained that develops only a slight color when exposed to direct sunlight during several days. Further purification according to methods described in the literature does not give a product which will remain colorless on standing.
4.
The low yield of
furfural from straw and other materials makes it desirable to extract the aqueous
furfural distillate after acid hydrolysis by a solvent heavier than water, such as
chloroform. This is done by adding a funnel to the end of the condenser so that the distillate passes directly into a layer of
chloroform contained in a receiver. Distillation is continued until the
chloroform layer does not increase. The
furfural is obtained by distillation of the
chloroform layer, and practically all the
chloroform is recovered (H. I. Waterman, private communication).
3. Discussion
Practically quantitative yields of
furfural are obtained when
pentoses are subjected to the action of
hydrochloric acid.
1 Carbohydrate materials such as corn cobs, wood, the hulls of oats, rice, peanuts, etc., when heated with steam under pressure or distilled with dilute
hydrochloric or sulfuric acids, yield appreciable quantities of
furfural. Practically all the
furfural now prepared technically is from oat hulls.
2This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
aldehyde
pentoses
hydrochloric or sulfuric acids
sulfuric acid (7664-93-9)
hydrochloric acid (7647-01-0)
sodium hydroxide (1310-73-2)
chloroform (67-66-3)
Furfural,
2-Furaldehyde (98-01-1)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved