Org. Synth. 1926, 6, 100
DOI: 10.15227/orgsyn.006.0100
TRIMYRISTIN
Submitted by G. D. Beal
Checked by H. T. Clarke and E. R. Taylor.
1. Procedure
In the container A (Fig. 30) is placed 1500 g. of crushed nutmegs (Note 1) moistened with ether (Note 2). A is a 3-l. inverted aspirator bottle connected by a 3-mm. glass tube to the efficient condenser C, and by 3-mm. tubing, one end of which is provided with a Soxhlet thimble to the 2-l. round-bottomed flask B. Flask B is connected by 3-mm. tubing of 75-cm. length to C. In B are placed 500 cc. of ether and a few chips of clay plate to prevent superheating. B is then heated on a steam cone so that the ether boils rapidly enough to reach the condenser C and to flow back through A.
Fig. 30.
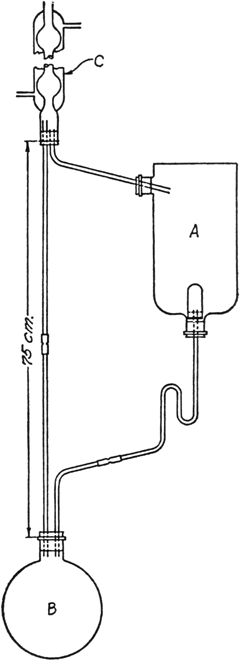
The extraction with
ether is continued until the ether leaving the insoluble solid is entirely colorless. This requires twenty-four to seventy-two hours, according to the state of subdivision of the nutmegs and the rate at which the
ether is passed through. The ethereal solution is then freed of a small quantity of entrained insoluble matter by filtering through a folded paper. This filtration may advantageously be completed in the type of extractor described on
p. 375. The clear solution is now entirely freed from ether by distillation on the
water bath. The residue weighs 640–690 g. On cooling it sets to a mass of crystals of
trimyristin which is filtered with suction
(Note 3) and washed with
225 cc. of cold 95 per cent ethyl alcohol in small portions. The product is now recrystallized from
3.5 l. of 95 per cent ethyl alcohol; it is stirred mechanically during cooling since the
trimyristin tends to separate as an oil at the outset
(Note 4). The crystallized
trimyristin is then filtered off by suction and washed with
350-400 cc. of 95 per cent alcohol in small portions. The crystals, which are colorless and practically odorless, melt at
54–55°. The yield is
330–364 g.
2. Notes
1.
If the nutmegs are crushed to No. 40 powder, as recommended by the author, the extraction is complete in twenty-four to forty-eight hours; in checking it was found more convenient merely to pass the nutmegs through a food chopper (whereby they were broken up into pieces the largest of which were 3–4 mm. across), when the extraction required sixty-six to seventy-two hours for completion.
West India nutmegs are less expensive than the East India variety, and there seems to be no difference in yields of trimyristin between the two kinds.
If nutmeg butter, a commercial fat obtained by the hot pressing of ground nutmegs, is available, the above extraction may be omitted. The only operation necessary is a double crystallization of the crude material from boiling 95 per cent alcohol. Since nutmeg butter is frequently adulterated with foreign fats, the purity of the product should be checked by the saponification number (232 for pure trimyristin).
2.
The nutmeg must first be moistened with ether; otherwise the extraction takes much longer. The author has found this apparatus to be generally satisfactory for the extraction of vegetable drugs with volatile solvents.
3.
The filtrate from the crude
trimyristin contains the odorous oils of the nutmeg. A further quantity of
trimyristin may be obtained from it by distilling with steam and recrystallizing the non-volatile residue twice from alcohol; but the amount is not commensurate with the trouble, and this operation is not advised unless the residues from at least 5 kg. of nutmegs are on hand.
4.
The alcohol may be distilled from the mother liquor of the recrystallization. The residue from this distillation may be added to the mother liquor of the first crystallization, which is then concentrated to the crystallization point. The crop of crystals thus obtained will usually require double recrystallization.
Alcohol recovered from the first mother liquor will contain too much volatile oil of nutmeg to be used for other purposes.
3. Discussion
Trimyristin can be isolated from nutmegs by ether extraction.
1
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
ethyl alcohol,
alcohol (64-17-5)
ether (60-29-7)
trimyristin (555-45-3)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved