Org. Synth. 1933, 13, 60
DOI: 10.15227/orgsyn.013.0060
METHYL IODIDE
[Methane, iodo-]
[(A) (From Methyl Alcohol)]
Submitted by Harold S. King
Checked by Louis F. Fieser
1. Procedure
Iodine is slowly washed by means of condensed liquid into a mixture of
methyl alcohol and phosphorus. The apparatus employed by the submitter,
Fig. 14, is rendered more flexible by the use of a
three-necked flask, as shown in
Fig. 15, and a further improvement is in the provision of a side tube for the addition of
iodine to the separatory funnel, A
(Note 1). In the following description reference is made to the simpler apparatus,
Fig. 14; the changes required for the modified assembly will be obvious.
Fig. 14
Fig. 15
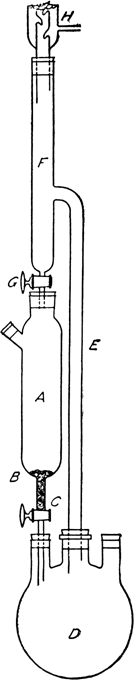
The cylindrical separatory funnel A has a volume of 1.2 l. and holds 2 kg. (15.75 gram atoms) of iodine crystals (Note 2). A piece of perforated platinum foil B, or a loose plug of glass wool, is placed in the bottom to prevent clogging of the stopcock by iodine crystals or solid impurities. Stopcock C is as large as 5 mm. in bore as a further precaution. For producing up to 4 kg. of methyl iodide, a 5-l. round-bottomed Pyrex flask D is used (Note 3). It is partly submerged in a water bath. Tube E, fitted into the reaction flask by a rubber stopper, is at least 2.5 cm. in internal diameter in order to allow the vapor to rise without interrupting the return of the excess distillate. Tube F is 4 cm. in diameter to allow for preliminary condensation. A space (13 cm.) is left above stopcock G as a reservoir for the distillate. Stopcock G has a bore of 2 or 3 mm. (Note 4). The bottom of the tube from this stopcock is flush with the bottom of the rubber stopper to the iodine container, so that the liquid will flow down the sides of the container instead of forming a channel through the middle of the iodine. Condenser H, 2.5 cm. in diameter and at least 200 cm. long, is attached by a rubber stopper and is well cooled with a strong stream of water (Note 5) and (Note 6).
In container A is placed
2 kg. of iodine, no crystals being allowed to fall below the platinum foil or glass wool. The
iodine is covered with part of
2 l. (50 moles) of methanol (Note 7), the rest of which is added to
200 g. each of red and yellow phosphorus (12.8 gram atoms) in reaction flask D. The apparatus is assembled as illustrated in
Fig. 14. The
water bath is heated to 70–75°; stopcock G is opened wide and stopcock C part way to allow the solution of
iodine to flow slowly into the reaction flask. By the time all the alcohol has been added, enough
methyl iodide will have been formed to start the refluxing. Stopcock C is then opened wide and stopcock G partly closed so that only a small stream of
methyl iodide flows through the iodine container. The temperature is adjusted so that little of the reflux flows back through tube E. This will require the progressive lowering of the temperature of the water bath as the reaction proceeds
(Note 8); a temperature of about 55° is sufficient to promote even boiling after the first portion of
iodine has been introduced. If for any reason refluxing becomes too violent, both stopcocks are closed. When the reaction has been brought under control, stopcock G is partly opened and then stopcock C part way, until any liquid in A has run out, after which C is opened wide. When all the
iodine has been extracted (about two and one-half hours) both stopcocks are closed, the separatory funnel is lowered with a rotary motion, filled with a second charge of
2 kg. (15.75 gram atoms) of iodine, and raised to its former position. Stopcock C is opened wide and stopcock G part way as before.
After the extraction of this second charge of iodine, the flask is cooled, and a condenser arranged for downward distillation is fitted in place of the special apparatus. To the lower end of the condenser is tightly attached an adapter dipping under a slush of ice and water. When all the methyl iodide has distilled (b.p. 40–42.5°) (Note 9), it is separated from the water, dried by shaking with anhydrous calcium chloride, filtered through glass wool, stored for use in a sealed flask or tubes, and kept in the dark (Note 10). The yield is 4150–4250 g. (93–95 per cent of the theoretical amount based on iodine), and the crude product is colorless and sufficiently pure for many purposes. Redistillation gives, with a 95 per cent recovery, a product boiling at 41.0–42.0°. Because of the high vapor pressure of methyl iodide, special precautions should be taken to prevent loss of material. Two different devices that have been found satisfactory are described in (Note 11) and (Note 12).
The discoloration of purified methyl iodide is greatly retarded by the addition of a drop of mercury, and traces of iodine in an old sample may be removed by shaking with mercury (Note 13). A very highly colored product is best washed with very dilute sodium thiosulfate, then with water, before drying.
With minor modifications, this method is suitable for the preparation of ethyl, n-propyl, n-butyl, n-amyl, isobutyl, and isoamyl iodides with yields of more than 90 per cent. A somewhat lower yield is obtained in the preparation of sec.-butyl iodide owing to the formation of hydrogen iodide.
2. Notes
1.
The upper opening of the funnel A
(Fig. 15) should be the same size as that of tube F, so that the funnel may be used as a receiver in the distillation described below
(Note 12).
2.
A funnel of this volume and shape is not essential but has been found convenient. Even if a
globular separatory funnel is used, the liquid flowing through it has been found to wet the walls.
3.
For larger quantities a
flask up to 12 l. in capacity may be substituted.
4.
The stopcock from a small, broken, separatory funnel serves the purpose.
5.
In hot weather there is usually some loss of
methyl iodide through the condenser. This can be recovered by a
trap made by bending the mouth end of a
50-cc. pipet into a U, which is stoppered into the end of the condenser, the delivery end being just below the surface of a slush of ice and water.
6.
In place of the very long condenser, the
checker used a
modified West condenser with staggered indentures, as shown in
Fig. 15. The jacket was 90 cm. in length, the inner tube was 2.2 cm. i.d., the opening at the top was 3.3 cm. i.d. At the top of this condenser was attached a
30-cm. bulb condenser which could be cooled with ice water. With the tap water at 5°, there was no difficulty in effecting complete condensation, and the trap described in
(Note 5) was not required. In warm weather it will probably be necessary to run the water to each condenser through a
large bottle of ice.
7.
The
methanol need not be absolute. In one experiment it was diluted with 10 per cent of its weight of water. The yield of
methyl iodide was identical with that of a check run in which
absolute methanol was used.
The refluxing starts more smoothly if the iodine crystals are covered with methanol. The alcoholic solution of iodine forms sufficient methyl iodide to start the refluxing at a lower temperature than would otherwise be necessary.
8.
The increased speed of the reaction is largely due to the fact that
iodine is more soluble in
methyl iodide than in
methanol and is therefore more rapidly introduced as the reaction progresses. The reaction is exothermic.
9.
It has been pointed out that this distillate is probably the constant-boiling mixture of
methyl iodide and
methyl alcohol which is composed of
93 per cent by weight of
methyl iodide and boils at
39°. The procedure given for recovering the
methyl iodide from the water-ice mixture is adequate. (Robert A. Dinerstein, private communication.)
10.
Caution is necessary in disposing of the residues left in the reaction flask because they contain
yellow phosphorus. They should be covered at once with water.
11.
One end of a
coil of 2-mm. glass tubing is sealed to the end of the inner tube of the condenser. The other end of the coil is sealed to a tube leading through the stopper of the
collecting vessel, which also carries a
Bunsen valve to prevent the entrance of moisture from the air. Both the coil and the collecting vessel are cooled with ice and salt, or with carbon dioxide snow.
12.
Another convenient assembly for the distillation is furnished by using the condenser shown in
Fig. 15, together with the separatory funnel, A. The
methyl iodide is distilled from a
3-l. flask through an
inverted U-tube (22 mm.); this brings the vapor down into the condenser, which is also clamped in a vertical position. The separatory funnel serves as the receiver and permits the easy removal of fractions. The side opening is equipped with a small condenser carrying a
calcium chloride tube. A simple and adequate method of recording the boiling temperature is to insert in the U-tube in an inverted position a
long thermometer with its head imbedded in a small cork, which rests on the bottom of the flask.
13.
Caution.
Methyl iodide in contact with
mercury when exposed to the sunlight readily forms
methylmercuric iodide, which is exceedingly poisonous.
[(B) (From Methyl Sulfate)]
Submitted by W. W. Hartman
Checked by John R. Johnson and H. J. Passino.
1. Procedure
A 3-l. three-necked flask is fitted with a thermometer, a separatory funnel, a liquid-sealed mechanical stirrer, and a small fractionating column connected with an efficient condenser set for downward distillation. The condenser leads to a receiving vessel cooled in ice water (Note 1). A solution of 800 g. (4.8 moles) of u.s.p. potassium iodide in 430 cc. of water is placed in the flask, 60 g. of calcium carbonate is added, and the mixture is heated to 60–65°, with stirring.
The temperature is kept at 60–65°, and 630 g. (473 cc., 5 moles) of "practical" methyl sulfate (Note 2) is added gradually through the separatory funnel. The rate of addition is such that the methyl iodide distils briskly; the addition requires about two hours.
After all the methyl sulfate has been added, the temperature is raised to 65–70° for about forty minutes to complete the distillation of the methyl iodide. The product is separated from a small amount of water, dried thoroughly over anhydrous calcium chloride, and decanted into a dry distilling flask. A few crystals of potassium iodide are added, and the material is distilled from a water bath. The yield of methyl iodide, boiling at 41–43°, is 615–640 g. (90–94 per cent of the theoretical amount) (Note 3).
2. Notes
1.
It is important to avoid loss of the volatile
methyl iodide. The notes to Procedure (
A) can be read with profit on this point.
2.
Methyl sulfate is extremely toxic. Contact with the liquid or inhaling the vapor should be avoided.
Ammonia is a specific antidote.
3.
Similar percentage yields are obtained with quantities of material up to ten times as large as those specified in the procedure.
3. Discussion
Methyl iodide has been prepared by the action of
methyl sulfate on an aqueous solution of
potassium iodide;
1 by the slow distillation of a mixture of
methanol with a large excess of constant-boiling
hydriodic acid;
2 by the electrolysis of an aqueous solution of
potassium acetate in the presence of
iodine or
potassium iodide;
3 by the action of an aqueous solution of
potassium iodide on
methyl p-toluenesulfonate;
4 and by the action of
methanol on a solution of
phosphorus pentaiodide in
methyl iodide.
5 The most generally employed method of preparing
methyl iodide has been the interaction of
methanol and
phosphorus triiodide (or a mixture of
iodine and
phosphorus, red, yellow, or both).
6 Many modifications of this method have been proposed and certain variants have been suggested for the preparation of the higher alkyl iodides which are applicable to the preparation of
methyl iodide.
7
The procedure described in (
A) is a modification of Walker's method.
8 The method of introducing a soluble substance gradually into a reaction flask, described above, is applicable to other reactions, such as the preparation of mercury dialkyls.
9The procedure described in (
B) can also be employed successfully with
iodoform as the iodine-containing starting material, if the
iodoform is first heated with alcoholic alkali.
10This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
yellow phosphorus
methyl alcohol and phosphorus
red and yellow phosphorus
ethyl, n-propyl, n-butyl, n-amyl, isobutyl, and isoamyl iodides
calcium chloride (10043-52-4)
ammonia (7664-41-7)
methyl alcohol,
methanol (67-56-1)
PHOSPHORUS (7723-14-0)
potassium iodide (7681-11-0)
sodium thiosulfate (7772-98-7)
mercury (7439-97-6)
calcium carbonate (471-34-1)
iodine (7553-56-2)
hydriodic acid,
hydrogen iodide (10034-85-2)
Methyl iodide,
Methane, iodo- (74-88-4)
methyl sulfate (75-93-4)
iodoform (75-47-8)
potassium acetate (127-08-2)
methylmercuric iodide
phosphorus pentaiodide
phosphorus triiodide (13455-01-1)
Methyl p-toluenesulfonate (80-48-8)
sec.-butyl iodide (513-48-4)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved