Org. Synth. 1953, 33, 3
DOI: 10.15227/orgsyn.033.0003
ALLOXANTIN DIHYDRATE
Submitted by R. Stuart Tipson
1
Checked by T. L. Cairns and F. S. Fawcett.
1. Procedure
An apparatus is assembled in the hood, as shown in
Fig. 1. In the
2-l. globe-shaped separatory funnel H (stopcock J closed) is placed 1.3 l. of deaerated water
(Note 1).
Three-holed rubber stopper G (bearing the stem of a 125-ml. separatory funnel C with stopcock D closed, a long inlet tube E, and a short outlet tube F) is inserted in the neck of H. In the neck of
C, a rubber stopper
B, provided with an inlet tube
A, is inserted.
H is flushed with
nitrogen (Note 2) admitted at
E. To the water is added
16.0 g. (0.1 mole) of alloxan monohydrate (Note 3), and the mixture is stirred by the flow of
nitrogen through
E until the
alloxan monohydrate has dissolved. The
nitrogen flow is discontinued, and
hydrogen sulfide (Note 4) is passed in at
E until the mixture is saturated with this gas and the aqueous solution is free from opalescence (about 2 hours).
Carbon disulfide (100 ml.) is now added, and the mixture is agitated for 5 minutes by means of the
hydrogen sulfide gas stream. The
carbon disulfide layer is then cautiously withdrawn through
J and discarded, and the aqueous solution is washed once more with
100 ml. of carbon disulfide which is separated and discarded. The
hydrogen sulfide flow is discontinued, and
nitrogen is passed in at
E for about 2 hours or until the gas emerging at
F gives no more than a faint test for
hydrogen sulfide with lead acetate paper.
Fig. 1. Apparatus for preparation of alloxantin dihydrate.
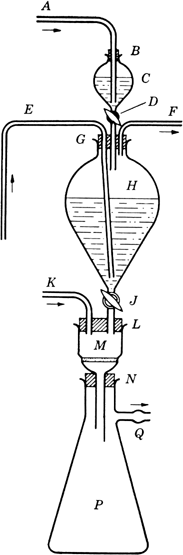
Tubes E and F are now closed, stopper B is loosened, and C is flushed out with nitrogen admitted through tube A. Deaerated water (100 ml.) is placed in C, which is then flushed with nitrogen. To this water is added alloxan monohydrate (16.0 g.; 0.1 mole), and the mixture is stirred with A, while the nitrogen stream is continued, until the solid has dissolved. B is now pushed down to give a tight fit, a slight pressure of nitrogen is applied (at A), F and D are opened, and the solution is allowed to pass from C into H. To wash out traces of alloxan, a further 10 ml. of water is placed in C and run into H. D is closed, and nitrogen is passed in at E and out of F until the solutions are thoroughly mixed. E and F are now closed, and the mixture in H is allowed to stand until crystallization is complete (overnight). In the meantime, the stem of funnel H is inserted in one hole of the two-holed rubber stopper L (also bearing inlet tube K); L is inserted in the mouth of M (a 150-ml., Pyrex Büchner funnel with coarse, fritted-glass septum); and the stem of M is inserted in the rubber stopper N placed in the neck of a 2-liter Büchner flask P (side arm, Q). To flush out M and P, nitrogen is passed in at K and out of Q. K and Q are now closed, F is opened, and a slight pressure of nitrogen is applied at F. Q and J are opened, and, if necessary, slight suction is applied at Q. When all the suspension has passed out of H, the nitrogen stream is continued for a few minutes, to remove as much as possible of the liquid clinging to the precipitate in M. Then F, J, and Q are closed. The Büchner funnel M and its contents are quickly removed, placed in a vacuum desiccator (preflushed with nitrogen), and dried to constant weight, at room temperature, over phosphorus pentoxide and soda-lime. The yield is 27–27.5 g. (84–85%) (Note 5), (Note 6), and (Note 7).
2. Notes
1.
Deaerated water is prepared as follows. A boiling stone is added to distilled water which is then boiled under reflux for at least 5 minutes; it is cooled in ice to room temperature under an atmosphere of oxygen-free
nitrogen.
2.
For preparation of moist, oxygen-free
nitrogen, the commercial gas is passed through (
a) a
500-ml. Drechsel bottle containing a fresh solution of
25 g. of sodium hydroxide plus 5 g. of pyrogallol in 250 ml. of deaerated water, (
b) a
reversed, empty 500-ml. bottle, and (
c) a
500-ml. bottle containing 250 ml. of deaerated water.
3.
Alloxan monohydrate from Eastman Kodak Company is satisfactory. It is dried to constant weight over
soda-lime and phosphorus pentoxide in the vacuum desiccator at room temperature. It should be colorless, and readily and completely soluble in 5 volumes of cold water. The sample employed assayed
99–100% alloxan monohydrate (p. 23) by Tipson and Cretcher's method.
24.
Commercial
hydrogen sulfide is passed through a
(reversed) empty 500-ml. Drechsel gas-washing bottle and then through 250 ml. of deaerated water in a similar bottle (not reversed).
5.
The solubility of
alloxantin dihydrate in water at room temperature is about 0.29 g. per 100 ml. of solution.
3 An additional 4 g. of product may be obtained by evaporation of the mother liquor to dryness at 25° under reduced pressure (
nitrogen atmosphere).
6.
The yield is slightly less if traces of crystals are left adhering to the inner walls of funnel
H. The
alloxantin obtained by dehydration of the product at 120–150° under reduced pressure for 2 hours melts with decomposition in 0.5 to 1 minute when placed in a block heated to 245°.
47.
The submitter reports that with minor modification the above
hydrogen sulfide reduction procedure can be applied to the preparation of the
dialuric acid monohydrate intermediate. The apparatus is assembled as in
Fig. 1 with the exception that funnel
C and its accessories are deleted. The above reduction procedure is followed initially, employing 500 ml. of deaerated water and
50 g. of alloxan monohydrate instead of the quantities shown above. After the saturation with
hydrogen sulfide (determined by weighing) and the first agitation with
carbon disulfide have been conducted as above, the funnel is assembled for filtration in an atmosphere of
hydrogen sulfide (rather than
nitrogen), and the suspension in
H is filtered through
M by manipulations analogous to those described. The colorless crystals of
dialuric acid monohydrate are washed on the filter with an additional
100 ml. of carbon disulfide added portionwise via
H, and, while wet with
carbon disulfide and
hydrogen sulfide, the crystals and funnel
M are transferred to a
shielded vacuum desiccator and dried over
soda-lime and
phosphorus pentoxide under high vacuum (
Dry Ice-cooled trap). The yield is
44–44.5 g. (
87–88%). Even at 300°, the compound exhibits no true melting or gas evolution. Heated at 2° per minute in an
aluminum block (initial temperature, 150°) it appears unchanged at 200°, turns faintly pink at 203–206°, and gradually becomes reddish brown (229–232°) and then purplish black at about 270°.
3. Discussion
This preparation is referenced from:
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
soda-lime
sodium hydroxide (1310-73-2)
hydrogen sulfide (7783-06-4)
nitrogen (7727-37-9)
carbon disulfide (75-15-0)
pyrogallol (87-66-1)
alloxantin (76-24-4)
Alloxan monohydrate (2244-11-3)
Alloxan (50-71-5)
Alloxantin dihydrate (6011-27-4)
phosphorus pentoxide (1314-56-3)
dialuric acid monohydrate (444-15-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved