Org. Synth. 1963, 43, 97
DOI: 10.15227/orgsyn.043.0097
4-PYRIDINESULFONIC ACID
Submitted by Russell F. Evans
1,2, Herbert C. Brown
1, and H. C. van der Plas
3.
Checked by James Cason and Taysir Jaouni.
1. Procedure
A. N-(4-Pyridyl)pyridinium chloride hydrochloride. In a 2-l. round-bottomed flask equipped with a ground joint (Note 2) is placed 395 g. (5.00 moles) of dry pyridine (Note 3). As this flask is cooled by swirling in a bath of cold water (Note 4), there is added during a few minutes 1190 g. (10.0 moles) of a good commercial grade of thionyl chloride (Note 1). After completion of the addition, the flask is protected by a drying tube, and the reaction mixture is allowed to stand at room temperature under a hood for 3 days. During this period, the color of the mixture changes from deep yellow through brown to black.
The flask is fitted with a Claisen head, and excess thionyl chloride is distilled at reduced pressure (water pump) and collected in a receiver cooled in a mixture of dry ice and acetone (Note 5). The flask is heated with a water bath that is slowly raised from room temperature to about 90°, then held at that temperature until no more distillation occurs and a black residue remains.
The black residue is cooled to 0°, and 100 ml. of ice-cold ethanol is added very cautiously to react with residual thionyl chloride. An additional 400 ml. of ice-cold ethanol is added, and the solid mass left at the bottom of the flask is broken up with the aid of a rod (Note 2). The resultant light-brown powder is collected by suction filtration, preferably on a sintered glass funnel, and washed with five 150-ml. portions of ethanol. The yield of crude N-(4-pyridyl)pyridinium chloride hydrochloride is 230–257 g. (40–45%). This product is very deliquescent and should be used immediately or stored over phosphorus pentoxide.
B. 4-Pyridinesulfonic acid. A 115-g. (0.50 mole) quantity of N-(4-pyridyl)pyridinium chloride hydrochloride is dissolved in 750 ml. of water in a 2-l. round-bottomed flask, and 378 g. (1.50 moles) of solid sodium sulfite heptahydrate is added cautiously. After the evolution of sulfur dioxide has ceased, the solution is gently heated under reflux in a nitrogen atmosphere for 24 hours. After slight cooling, 20 g. of charcoal is added to the mixture, and it is heated under reflux for an additional hour. The resultant mixture is filtered through a fluted paper, the filtrate is evaporated to dryness on a steam bath under reduced pressure, and the residue is air-dried at 100–110° (Note 6). This solid is now continuously extracted with absolute ethanol for 24 hours in a Soxhlet apparatus. The alcohol is distilled from the extract on a steam bath, and the crude sodium 4-pyridinesulfonate is dissolved in about 160 ml. of hot water. After 320 ml. of 12N hydrochloric acid has been added with mixing, the solution is cooled to room temperature. The precipitate of sodium chloride is filtered, and the filtrate is evaporated to dryness under reduced pressure on a steam bath. Crystallization of the residue from 600 ml. of 70% aqueous ethanol yields 27–30 g. of colorless crystals of 4-pyridinesulfonic acid, m.p. 313–315° (dec.). Concentration of the mother liquor affords about 10 g. of additional product which is less pure. The total yield is 36–40 g. (45–50%) (Note 7). Recrystallization from 70% aqueous ethanol affords a purer specimen, m.p. 317–318° (dec.). (Note 8).
2. Notes
1.
Although
N-(4-pyridyl)pyridinium chloride hydrochloride is formed by reaction of
pyridine with
thionyl chloride, followed by treatment with
ethanol, the intermediates involved in the reaction have not been well established. It has been suggested
4,5 that
1 mole of thionyl chloride converts
2 moles of pyridine to the compound. This intermediate would be further oxidized by
thionyl chloride and solvolyzed by
ethanol to the pyridinium chloride hydrochloride. According to this reaction route, the stoichiometric ratio of
pyridine to
thionyl chloride for the overall process would be about 1:1. Varying ratios of
thionyl chloride have been used
6,7,8 and varying yields of the product have been reported, ranging from
60% of crude product
7 to
48% of recrystallized product.
8 In one run in which the checkers used one-half the specified amount of
thionyl chloride, the yield was unaffected. The submitters report yields in the range
58–62% by the procedure described here.
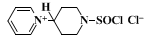
2.
Thionyl chloride attacks rubber so rapidly that all-glass apparatus is highly desirable for this procedure. Since breaking up the residual product in a flask results in a high mortality of flasks, the checkers preferred a distilling vessel with a removable top of the type used with
vacuum desiccators (e.g., Corning Glass Works, No. 3480).
3.
Since moisture reacts with
thionyl chloride to give
hydrogen chloride, which forms the salt of pyridine and thus inactivates it, the
pyridine should be dried over
barium oxide for 24 hours, then distilled under anhydrous conditions shortly before use.
4.
Provided that this addition is carried out rapidly, ingress of moisture is not significant, and more complicated apparatus is not recommended.
5.
Since
thionyl chloride ruins all rubber tubing with which it comes in contact, efficient cooling of the receiver is recommended.
6.
Alternatively, to decrease the time required to complete drying at 100–110°, the moist solid residue may be triturated with
chloroform and the
chloroform distilled from the steam bath. The checkers used a
vacuum oven for drying.
7.
The submitters report yields in the range
63–70%.
8.
Because the sulfonic acid melts with decomposition, the value observed for the melting point is highly dependent on the rate of heating of the sample.
9,10,11,12
3. Discussion
The preparation of
N-(4-pyridyl)pyridinium chloride hydrochloride follows the procedure of Koenigs and Greiner,
6 while the preparation of the sulfonic acid is a modification of a patent procedure.
134-Pyridinesulfonic acid has been prepared by oxidation of
4-pyridinethiol with
hydrogen peroxide in
barium hydroxide solution,
9 with
hydrogen peroxide in
glacial acetic acid,
10 with
nitric acid-chlorine or
nitric acid-chlorine-hydrochloric acid mixtures,
11 and with
nitric acid alone.
10,12,14 The latter reaction gives a mixture of
4-pyridinesulfonic acid and other products, e.g.,
di-4-pyridyl disulfide dinitrate, and this has led to some confusion in the literature.
10,11,12,14 4-Pyridinesulfonic acid has also been obtained from its N-oxide derivative by reduction of the N-oxide group with
iron and
acetic acid15 or catalytically.
16
Sodium 4-pyridinesulfonate has been formed by the oxidation of
4-pyridinethiol with
hydrogen peroxide in
sodium hydroxide solution,
17,18 and from the reaction of
4-chloropyridine with aqueous
sodium sulfite.
19 The salt has been converted to the free acid by treatment with a cation-exchange resin
10,11 or with
sulfuric acid.
11
4. Merits of the Preparation
This is the most convenient preparation of 4-pyridinesulfonic acid, a useful intermediate for the synthesis of various pyridine derivatives.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
nitric acid-chlorine-hydrochloric acid
ethanol (64-17-5)
sulfuric acid (7664-93-9)
hydrogen chloride,
hydrochloric acid (7647-01-0)
acetic acid (64-19-7)
sodium sulfite (7757-83-7)
sodium hydroxide (1310-73-2)
thionyl chloride (7719-09-7)
chloroform (67-66-3)
iron (7439-89-6)
nitric acid (7697-37-2)
sodium chloride (7647-14-5)
sulfur dioxide (7446-09-5)
barium oxide
nitrogen (7727-37-9)
acetone (67-64-1)
pyridine (110-86-1)
hydrogen peroxide (7722-84-1)
barium hydroxide (17194-00-2)
4-Pyridinesulfonic acid (5402-20-0)
N-(4-Pyridyl)pyridinium chloride hydrochloride (5421-92-1)
sodium sulfite heptahydrate
sodium 4-pyridinesulfonate
4-pyridinethiol (4556-23-4)
nitric acid-chlorine (14545-72-3)
4-chloropyridine (626-61-9)
phosphorus pentoxide (1314-56-3)
di-4-pyridyl disulfide dinitrate
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved