Org. Synth. 1978, 58, 67
DOI: 10.15227/orgsyn.058.0067
2,3-DICYANOBUTADIENE AS A REACTIVE INTERMEDIATE BY in situ GENERATION FROM 1,2-DICYANOCYCLOBUTENE: 2,3-DICYANO-1,4,4a,9a-TETRAHYDROFLUORENE
[2,3-Fluorenedicarbonitrile, 1,4,4a,9a-tetrahydro-]
Submitted by D. Bellus
1, H. Sauter, and C. D. Weis.
Checked by A. J. Arduengo and William A. Sheppard.
1. Procedure
Caution! Benzene has been identified as a carcinogen. OSHA has issued emergency standards on its use. All procedures involving benzene should be carried out in a well-ventilated hood, and glove protection is required.
A. 1-Chloro-1,2-dicyanocyclobutane (1). A 2-l., three-necked flask is equipped with a mechanical stirrer, a 500-ml. pressure-equalizing funnel, and an efficient reflux condenser provided with a gas-outlet tube connected by plastic tubing to a conical funnel inverted over a 5-l. beaker containing aqueous sodium hydroxide, for absorption of the evolved hydrogen chloride. The flask is charged with 562 g. (2.70 moles) of phosphorus pentachloride and 750 ml. of carbon tetrachloride. The rapidly stirred suspension is heated to reflux, and a solution of 159 g. (1.50 moles) of 1,2-dicyanocyclobutane (Note 1) in 120 ml. of chloroform is added dropwise over a period of 40 minutes (Note 2). After addition is complete, the solvents and the phosphorus trichloride formed during the reaction are removed by distillation at 100–150 mm. over a period of 40–60 minutes, with a bath temperature not exceeding 80° (Note 2). The residual liquid is cooled to room temperature and dissolved in 400 ml. of diethyl ether (Note 3). The etheral solution is placed in a 500-ml.dropping funnel and added over a period of 3 hours to a stirred slurry of 1.7 kg. (12 moles) of sodium hydrogen carbonate, 800 g. of crushed ice, and 500 ml. of water. During the addition the temperature is maintained between −5° and 0° with an external ice–salt bath, and after the addition, stirring is continued for 1 hour at the same temperature. The precipitated salts are removed by suction filtration through a sintered-glass funnel of medium porosity and are thoroughly washed with 300 ml. of ether. The organic layer is separated, and the aqueous filtrate extracted with three 200-ml. portions of ether. The combined ether extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated on a rotary evaporator, yielding 166–174 g. (79–83%) of a yellow oil consisting of an isomeric mixture of crude cyclobutane 1 (Note 4).
B.
1,2-Dicyanocyclobutene (2). A
2-l., three-necked, round-bottomed flask fitted with a 500-ml. pressure-equalizing dropping funnel, a mechanical stirrer, and a reflux condenser protected from moisture by a
calcium chloride tube is charged with
131 g. (1.30 moles) of triethylamine (Note 5) and
400 ml. of benzene. The stirred solution is heated to gentle reflux, and a solution of
168.5 g. (1.199 moles) of crude cyclobutane 1 in
200 ml. of benzene is added dropwise over a period of 30 minutes. After addition is complete, the mixture is stirred under reflux for an additional 2 hours; the precipitated
triethylamine hydrochloride is filtered from the cold solution and washed with
150 ml. of benzene. The combined filtrates are washed twice with 200-ml. portions of water and evaporated using a
water aspirator at a bath temperature of 35°. The residue is distilled, yielding
105–110 g. (
83–87%) of crude cyclobutene
2, b.p.
55–60° (0.06 mm.) (Note 6).
C.
2,3-Dicyano-1,4,4a,9a-tetrahydrofluorene (3). A
100-ml., round-bottomed flask equipped with a reflux condenser under
nitrogen pressure is charged with
10.4 g. (0.100 mole) of crude cyclobutene 2,
23.3 g. (0.201 mole) of indene (Note 7), and
0.3 g. of hydroquinone. The reaction mixture is stirred and heated at 150° for 4 hours under
nitrogen. The reflux condenser is replaced by a still head, and
6.3 g. (0.053 mole) of indene is distilled from the flask at a bath temperature of about 95° (11 mm.)
(Note 8). The dark-colored reaction mixture is transferred to a
500-ml., round-bottomed flask, diluted with
200 ml. of benzene followed by
1 g. of decolorizing carbon, and the resulting mixture is refluxed for 2 hours. After the mixture is cooled to room temperature, the
carbon is removed by filtration, and the
benzene is distilled. The residual oily residue solidifies on standing and is recrystallized from
45 ml. of ethanol, yielding
15.4–15.9 g. (
70–72%) of crystalline fluorene
3, m.p.
98.5–100° (Note 9).
2. Notes
1.
1,2-Dicyanocyclobutane (cis- and trans-isomer mixture) was purchased from Aldrich Chemical Company, Inc., and used without further purification.
2.
The addition and distillation must be accomplished within the specified period of time; otherwise the amount of dichlorinated
1,2-dicyanocyclobutane increases considerably. The submitters found that an
80% molar excess of phosphorus pentachloride is optimum. A molar excess less than specified (under given experimental conditions) gives considerable unreacted starting material. Under forcing experimental conditions, such as longer reaction times and/or higher temperatures, the starting material can be completely consumed, even with less than
80% molar excess of phosphorus pentachloride, but a considerable amount of dichlorinated products is formed.
3.
The checkers found that, because of the time required for completion of this step, a convenient modification is to cool the ether solution to −78° in dry ice and store overnight at −78°. A solid complex of
phosphorus pentachloride and cyclobutane
1, m.p.
88–91°, precipitates in the cold
ether solution. This complex may not redissolve on warming to room temperature, but the suspension in
ether can be used to proceed with the second half of Step A.
4.
The checkers obtained the cyclobutane
1 as a colorless crystalline solid, m.p.
45–47° (a mixture of major and minor isomers), that is relatively free of
1,2-dichloro-1,2-dicyanocyclobutane. The product had the following spectral properties:
1H NMR (CDCl
3) δ (multiplicity): 2.35–3.15 (m), 3.40–4.20 (m);
13C NMR (CDCl
3), δ: major isomer 21.58 (t), 38.41 (d), 37.24 (t), minor isomer 22.48 (t), 36.72 (d), 36.91 (t).
5.
Reagent grade triethylamine was dried over
sodium hydroxide and distilled before use (b.p.
88–89°).
6.
The checkers obtained yields of
115 g. (
92%) on a 1.2 mole scale and
94 g. (
90%) on a 1.0 mole scale.
The product was analyzed by GC on a 1.23 m. × 0.65 cm. stainless-steel column of SE-52 on Varoport 30, which was heated to 150° and swept with helium at 60 ml. per minute. Retention times for the various components (minutes) are: cyclobutene 2, (2.6); trans-1,2-dicyanocyclobutane(3.6); two isomeric 1,2-dichloro-1,2-dicyanocyclobutanes (5.1 and 5.9, respectively); cis-1,2-dicyanocyclobutane (9.8).
For most synthetic purposes, such as [4 + 2]- and [2 + 2]-cycloadditions,
2,3,4,5,6,7 ring-opening reactions,
2,8 and hydrolytic reactions,
2 this crude
cyclobutene 2, which contains approximately 1–3.5% of isomeric mixtures of 1,2-dichloro-1,2-dicyanocyclobutanes, can be used satisfactorily without further purification. Pure cyclobutene
2 can be prepared by treatment of the crude product with
Raney cobalt, thereby removing residual quantities of isomeric 1,2-dichloro-1,2-dicyanocyclobutanes. In a typical experiment the crude product is placed in a
250-ml., round-bottomed flask and stirred with
10 g. of Raney cobalt for 4 hours at 70° under
nitrogen. Distillation directly from the reaction vessel without filtering off the metal slurry yields
94–98 g. (
60–63%) of cyclobutene
2 as a colorless liquid,
nD20 1.4926,
d224 1.033. The
Raney cobalt used by the submitters was obtained from Fluka A G, Buchs, Switzerland, as a suspension in water, and washed with
tetrahydrofuran before use.
Raney nickel and
nickel tetracarbonyl, respectively, are also good dechlorinating reagents. The use of
Raney cobalt, however, diminishes the danger of self-ignition during the preparation procedure. The spectral properties are as follows: IR (neat) cm
−1: 3002, 2957, 2230, 1612, 1422, 1251, 1169, 1003, and 623; UV (CH
3OH) nm. max. (log ε): 235 (4.06), 247 sh (3.90);
1H NMR (CDCl
3), δ (multiplicity): 2.91 (s).
7.
The
indene used by the submitters was "practical grade," purchased from Fluka A G, Buchs, Switzerland. The
indene used by the checkers was purchased from Aldrich Chemical Company, Inc. Both were distilled (b.p.
60–65°, 11 mm.) before use.
8.
Recovered
indene may be used for the next batch without further purification.
9.
Fluorene
3 has the following spectral properties: IR (KBr) cm
−1: 2222, 1608, 1479, 1440, 773, and 742; UV (C
2H
5OH) nm. max. (log ε): 216 (4.10), 2.34 (3.99), 261 (3.16), 267 (3.11), and 274 (3.07); mass spectrum
m/e: 220 (
m+) and 116 (base peak);
1H NMR (100 MHz., CDCl
3): two complex multiplets for the aromatic and aliphatic protons (360 MHz., CDCl
3), δ (multiplicity, coupling constant
J in Hz., number of protons, assignment): 2.10 [d,
J = 18; d,
J = 7; and t,
J = 3; one H of CH
2(1) or one H of CH
2(4)], 2.55–2.70 [m, one
H of CH
2(1), one
H of CH
2(4), one
H of CH
2(9) and CH(9a)], 2.88 [d,
J = 18; d,
J = 7; d,
J = 3; and d,
J = 1.5; one
H of CH
2(1) or one
H of CH
2(4)], 3.11 [d,
J = 15; d,
J = 6, one
H of CH
2(9)], 3.42 [d,
J = 5; t,
J = 7, C
H(4a)], 7.20 (m, 4, aromatic
H);
13C NMR (CDCl
3), δ (assignment): 143.8 [
C(8a)], 141.3 [
C(4b)], 127.6, 127.2, and 125.6 [
C(6),
C(7), and
C(8), not assigned individually], 126.6 and 125.9 [
C(2) and
C(3), not assigned], 123.2 [
C(5)], 115.9 [two nitrile carbons], 40.2 [
C(4a)], 38.4 [
C(9)], 35.4 [
C(9a)], 30.7 and 29.7 [
C(1) and
C(4), not assigned].
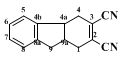
3. Discussion
Three syntheses of
1,2-dicyanocyclobutene (
2) have been previously described. The first involves dehydration of
cyclobutene-1,2-dicarboxamide, with no specified yield.
9 The second procedure involves a concomitant chlorination and catalytic dehydrochlorination of
1,2-dicyanocyclobutane in the gas phase, yielding
1,2-dicyanocyclobutene (
2) in a mixture of several other products.
10 The third method consists of dechlorination of
1,2-dichloro-1,2-dicyanocyclobutane using metals, such as
zinc copper couple,
11 Raney nickel,
11 and, especially,
Raney cobalt.
2 In comparison with the third synthesis, the overall yield of the present procedure is 5–10% higher. Furthermore, the reaction is performed in less time and utilizes considerably cheaper reagents.
Pure, crystalline
2,3-dicyanobutadiene has been prepared in high yield by gas-phase thermolysis of cyclobutene (
2).
2,8 Analogously, thermolysis of derivatives of
cyclobutene-1,2-dicarboxylic acid appears to represent a general procedure for the synthesis of derivatives of
butadiene-2,3-dicarboxylic acid, of high purity.
2,12 These butadienes take part in [4 + 2]-cycloaddition reactions either as reactive dienes
2,13,14 or as reactive dienophiles.
2,14 In the pure state, however, they tend to polymerize, and even crystalline
2,3-dicyanobutadiene slowly polymerizes, yielding a highly cross-linked polymer without losing its original crystal form. A [2 + 4]-dimer of a
2,3-dicyanobutadiene is also formed by heating the
dicyanocyclobutene in solution with a polymerization inhibitor.
2 Monomeric derivatives of
butadiene-2,3-dicarboxylic acid cannot be prepared in solution because of rapid dimerization.
8,14The present procedure,
in situ generation and trapping of
2,3-dicyanobutadiene in the presence of olefins, overcomes these problems and affords [4 + 2]-cycloadducts in good yields, particularly in the case of olefins possessing a strained double bond.
2 Substituted 1,2-dicyanocyclohexenes, prepared by the
in situ [4 + 2]-cycloadditions, can be dehydrogenated to new aromatic
ortho-dinitriles. For example,
2,3-dicyanofluorene is prepared in
56% yield by heating
2,3-dicyano-1,4,4a,9a-tetrahydrofluorene (3) at 200° in
dimethylmaleate in the presence of
5% palladium on charcoal. Other aromatic
ortho-dinitriles have also been prepared by this method.
2 Because
2,3-dicyanobutadiene is an electron-deficient diene, it does not react with electron-deficient olefins, such as
maleic anhydride and
fumaronitrile2,8 using this procedure. However, by generating the
dicyanobutadiene in refluxing
chlorobenzene in the presence of
maleic anhydride and
2,5-di-tert-butylbenzoquinone, as an inhibitor, the [2 + 4]-cyclic dicarbonitrile adduct, m.p.
201–202.5°, was formed in a yield of
38%.
15
TABLE I
[4 + 2]-CYCLOADDITION REACTIONS OF 2,3-DICYANOBUTADIENE FORMED in situ FROM 1,2-DICYANOCYCLOBUTENE2
|
|
Olefin
|
Product
|
Yield (%)a
|
Temperature (°C)
|
Time (hours)
|
|
|
Norbornadiene
|
4,5-Dicyanotricyclo-[6.2.1.02,7]undeca-4,9-diene
|
79b
|
150
|
12
|
|
Acenaphthylene
|
8,9-Dicyano-6b,7,10,10a-tetrahydrofluoranthene
|
77
|
138
|
48
|
|
Cyclopentene
|
3,4-Dicyanobicyclo[4.3.0]non-3-ene
|
65
|
135
|
16
|
|
Ethylene
|
1,2-Dicyanocyclohexene
|
58
|
135
|
16
|
|
(E)-Stilbene
|
1,2-Dicyano-4,5-diphenylcyclohexene
|
32
|
138
|
24
|
|
Butyl vinyl ether
|
1,2-Dicyano-4-butoxycyclohexene
|
28
|
155
|
16
|
|
(E)-1,2-Dichloroethylene
|
1,2-Dicyano-4,5-trans-dichlorocyclohexene
|
8
|
135
|
16
|
|
2-Vinylpyridine
|
1,2-Dicyano-4-(2 -pyridyl)cyclohexene
|
5
|
138
|
48
|
|
a Yields of analytically pure products are given.
|
b A 70:24 mixture of exo and endo isomers. Some 2:1 cycloadduct was also isolated (2.4% yield).
|
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Raney cobalt
zinc copper couple
ethanol (64-17-5)
hydrogen chloride (7647-01-0)
Benzene (71-43-2)
ether,
diethyl ether (60-29-7)
sodium hydroxide (1310-73-2)
phosphorus pentachloride (10026-13-8)
chloroform (67-66-3)
hydroquinone (123-31-9)
sodium hydrogen carbonate (144-55-8)
carbon tetrachloride (56-23-5)
nitrogen (7727-37-9)
Raney nickel (7440-02-0)
decolorizing carbon,
carbon (7782-42-5)
chlorobenzene (108-90-7)
palladium (7440-05-3)
phosphorus trichloride (7719-12-2)
ethylene (9002-88-4)
Triethylamine hydrochloride (554-68-7)
magnesium sulfate (7487-88-9)
Cyclopentene (142-29-0)
indene (95-13-6)
Tetrahydrofuran (109-99-9)
maleic anhydride (108-31-6)
triethylamine (121-44-8)
Fumaronitrile (764-42-1)
helium (7440-59-7)
norbornadiene
cyclobutene (822-35-5)
2,3-DICYANOBUTADIENE
1,2-Dicyanocyclobutene,
dicyanocyclobutene (3716-97-0)
1,2-dicyanocyclobutane
1,2-dichloro-1,2-dicyanocyclobutane
nickel tetracarbonyl
cyclobutene-1,2-dicarboxamide
cyclobutene-1,2-dicarboxylic acid
butadiene-2,3-dicarboxylic acid
2,3-dicyanofluorene
dimethylmaleate
dicyanobutadiene
4,5-Dicyanotricyclo-[6.2.1.02,7]undeca-4,9-diene
Acenaphthylene (208-96-8)
3,4-Dicyanobicyclo[4.3.0]non-3-ene
1,2-Dicyanocyclohexene
1,2-Dicyano-4,5-diphenylcyclohexene
Butyl vinyl ether (111-34-2)
1,2-Dicyano-4-butoxycyclohexene
2-Vinylpyridine (100-69-6)
1,2-Dicyano-4-(2 -pyridyl)cyclohexene
(E)-stilbene (103-30-0)
2,5-di-tert-butylbenzoquinone
1-Chloro-1,2-dicyanocyclobutane (3716-98-1)
2,3-Dicyano-1,4,4a,9a-tetrahydrofluorene,
2,3-Fluorenedicarbonitrile, 1,4,4a,9a-tetrahydro- (52477-65-3)
cis-1,2-dicyanocyclobutane
(E)-1,2-Dichloroethylene (156-60-5)
1,2-Dicyano-4,5-trans-dichlorocyclohexene
trans-1,2-dicyanocyclobutane
8,9-Dicyano-6b,7,10,10a-tetrahydrofluoranthene
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved