Org. Synth. 1983, 61, 98
DOI: 10.15227/orgsyn.061.0098
1,3-OXAZEPINES VIA PHOTOISOMERIZATION OF HETEROAROMATIC N-OXIDES: 3,1-BENZOXAZEPINE
Submitted by Angelo Albini, Gian Franco Bettinetti, and Giovanna Minoli
1.
Checked by Patrick MacManus and Robert M. Coates.
1. Procedure
Caution! 3,1-Benzoxazepine is a strong lacrimator and a moderate skin irritant. The preparation should be carried out in a well-ventilated hood. The apparatus should be shielded to avoid exposure to ultraviolet light.
Benzene has been identified as a carcinogen; OSHA has issued emergency standards on its use. All procedures involving benzene should be carried out in a well-ventilated hood, and glove protection is required.
Irradiation is carried out in a round-bottomed, cylindrical, Pyrex vessel (Note 1) equipped with a Pyrex immersion well (Note 2), nitrogen inlet, distillation sidearm, a small sidearm fitted with a rubber septum for removing aliquots, and a magnetic stirring bar. The flask is charged with 12 g (0.066 mol) (Note 3) of quinoline N-oxide dihydrate (Note 4) and 1.3 L of dry benzene (Note 5). The mixture is stirred and heated to boiling with a heating mantle as a slow stream of nitrogen (Note 6) is bubbled into the vessel. Benzene is distilled through the sidearm until the distillate is perfectly clear (Note 7). The lamp (Note 8) is placed in the immersion well, water is circulated through the cooling jacket, and the nitrogen flow is adjusted as necessary to maintain an outward flow of gas while the light yellow solution cools to room temperature (Note 9). The solution is stirred vigorously (Note 10) and irradiated for 2.5–3 hr, at which time the N-oxide is largely consumed (Note 11). The orange solution is transferred to a 1-L, round-bottomed flask, and the benzene is removed by rotary evaporation at room temperature. The red-orange oily residue, which contains some solid, is extracted with three 40-mL portions of dry cyclohexane, the combined cyclohexane extracts are evaporated under reduced pressure at room temperature, and the extraction operation is repeated on the oil thus obtained (Note 12). Evaporation of the combined cyclohexane extracts affords 6.1–6.3 g (63–65%) of crude 3,1-benzoxazepine (Note 13). Bulb-to-bulb distillation in a Kugelrohr apparatus at 0.2 mm with an oven temperature of 80°C affords 4.7–4.8 g (49–50%) of 3,1-benzoxazepine as a pale yellow oil, nD24 1.6074 (Note 14) and (Note 15).
2. Notes
1.
The apparatus used by the checkers for irradiations at a 9-g scale was 33 cm high and 9 cm in diameter and had a 60/50 T joint at the top for the immersion well. The joints for the distillation and sampling sidearms were 24/40 and 14/20, respectively. The gas inlet was located about 6 cm from the bottom of the vessel to accommodate the use of a heating mantle. A disk of coarse, sintered glass was sealed into the gas inlet near its point of attachment. The capacity of the vessel with the immersion well in place was ca. 900 mL. The submitters used a similar but flat-bottomed apparatus of 1.2-L capacity. The flat-bottomed vessel facilitates vigorous stirring, but it does not fit as well into the heating mantle and may therefore be somewhat hazardous to use.
2.
The checkers used a
Vycor immersion well and a
Pyrex filter sleeve. The immersion well, 450-W
mercury lamp, and the requisite transformer are available from Hanovia Lamp Division, Canrad-Hanovia Inc., 100 Chestnut Street, Newark, NJ 07105.
3.
The checkers carried out the irradiation on a 9-g scale in
750 mL of benzene after azeotropic distillation.
4.
Quinoline N-oxide dihydrate is supplied by Aldrich Chemical Company, Inc. and EGA Chemie KG, Steinheim/Albuch, Germany. The submitters prepared the compound by the procedure of Hayashi
2 with minor modifications. The water of hydration may be removed under reduced pressure in a drying pistol. However, since the anhydrous
N-oxide is very hygroscopic, the submitters have found that it is more expedient to use the dihydrate and remove the water by azeotropic distillation in the irradiation vessel.
5.
The checkers dried the
benzene by distillation from
calcium hydride immediately before use. The submitters report that
toluene may be used instead of
benzene; however, since the product is not very thermally stable, they advise that the
toluene should be evaporated without heating above 35–40°C during the isolation.
6.
Other dry gases may be used. The submitters report that the reaction is not quenched by
oxygen.
7.
A total of ca. 150–300 mL was collected. The distillation time may be reduced by insulating the vessel and sidearm with glass wool.
8.
The submitters used a Helios Italquartz 500-W lamp which has emission characteristics similar to those of the Hanovia 450-W medium pressure
mercury lamp used by the checkers.
9.
The checkers noticed that a thin film of oil that was evidently
quinoline N-oxide deposited on the surface of the immersion well and irradiation vessel during cooling.
10.
Vigorous stirring is essential for optimum yields. The checkers obtained lower isolated yields (ca. 32–33%) in two runs in which a relatively slow stirring rate was employed. The low yields were probably caused in part by deposition of oil on the surface of the immersion well and the resulting interference with the transmission of UV light into the solution.
11.
The submitters emphasize the importance of terminating the irradiation before all of the
N-oxide is consumed. Overirradiation gives rise to a more complicated mixture of products from which the product can no longer be isolated by the simple extraction procedure described.
The progress of the irradiation was determined by the checkers by proton NMR analysis. At appropriate intervals 5-mL aliquots were removed, the solvent was evaporated, and hexamethylbenzene was added as an internal standard. The ratio of N-oxide, benzoxazepine, and hexamethylbenzene was determined from integration of the resonances at δ 2.26 (s, 18H), 5.55 (d, 1H, J = 6), and 8.46 (d, 1H, J = 6), respectively, in chloroform-d. After 2.5–3 hr of irradiation the amount of benzoxazepine present was ca. 60–68% of theoretical and ca. 10% of starting N-oxide remained.
The submitters followed the course of the irradiation by TLC analysis on silica gel with 5% (v/v) methanol in chloroform as developing solvent. Since some by-products have R1 values coincident with the N-oxide, this spot will not completely disappear and caution must be exercised to avoid overirradiation.
12.
This extraction procedure separates most of the
carbostyril, that is, 2(1H)-quinolinone, which is formed to the extent of ca.
20% in the irradiation. The submitters have isolated the
carbostyril by-product by crystallization of the extraction residue from
95% ethanol in runs carried out to high conversion. Alternatively, the
carbostyril may be isolated by chromatography of the crude product on
silica gel with
5% methanol-chloroform as eluant. However, the
benzoxazepine cannot be obtained by this method since it undergoes hydrolysis during the chromatography.
13.
The purity of the crude product is about 90% according to NMR analysis, the remaining material being mostly unchanged
N-oxide.
14.
The spectral properties of the product are as follows: IR (liquid film) cm
−1: 1665 (C=N), 1630 (C=C), 1480 (sharp), 1440 (sharp), 1035 (strong), 765 (strong);
1H NMR (CDCl
3) δ: 5.55 (d, 1 H,
J = 6, C
H=CH-O), 5.84 (d, 1 H,
J = 6, CH=C
H-O), 6.44 (s, 1 H, O-CH=N), 6.96 (m, 4 H, aromatic protons).
15.
Samples of the product stored in tightly stoppered flasks in a freezer at −20°C for several weeks showed no sign of decomposition.
3. Discussion
The preparation of
3,1-benzoxazepines by photochemical isomerization of
quinoline N-oxides constitutes a rather general entry into this class of seven-membered heterocycles. Since the structure of the photoisomer of
2-phenylquinoline N-oxide was first recognized as
2-phenyl-3,1-benzoxazepine by Buchardt et al.,
3 the scope of this method for oxidative ring expansion of six-membered heterocyclic
N-oxides to
1,3-oxazepines has been extensively explored.
4 5 For example, irradiation of 2-cyano-, 2-phenyl-, and 2-methoxyquinoline
N-oxides affords the corresponding 2-substituted 3,1-benzoxazepines in 70–90% yield.
6 7 8 9 However, isolation of the moisture-sensitive parent compound was only recently accomplished in the submitters' laboratories.
10Related
1,3-oxazepines have been obtained from irradiation of many other heterocyclic
N-oxides including
pyridine N-oxides, isoquinoline N-oxides, quinoxaline N-oxides, quinazoline N-oxides, phenanthridine N-oxides, benzophenazine N-oxides, and acridine N-oxides.
4 5 However, the reported yields are variable and have generally been higher for phenyl and other aryl-substituted derivatives. This procedure is also satisfactory for
1,3-benzoxazepine (from
isoquinoline N-oxide) and, in general, for oxazepines not substituent-stabilized.
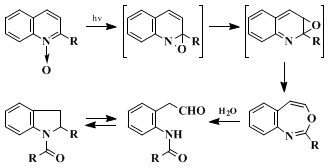
A mechanism involving initial cyclization to an oxaziridine, [1,5] sigmatropic rearrangement to an imino epoxide, and electrocyclic ring opening was originally proposed for the photochemical isomerization.
4 5 However, since later attempts to detect intermediates by flash photolysis were unsuccessful,
11 12 ground-state oxaziridines, if formed at all, must have exceedingly short lifetimes. The benzoxazepines undergo facile hydrolysis to
o-(N-acylamino)phenylacetaldehydes, which frequently exist as the cyclic carbinol amide tautomers. If water is present during the irradiation from use of the
N-oxide hydrate or moist solvent, the hydrolysis products may be isolated instead of the
benzoxazepine. Dehydration of the carbinol amide to
N-acyl indoles may also occur during irradiation and/or purification of the products. The formation of
carbostyrils is sometimes an important competing reaction in the irradiation of
quinoline N-oxides, and this by-product is, in fact, formed to the extent of ca.
20% in the present procedure. The use of polar protic solvents such as water or alcohols favors
carbostyril formation in contrast to aprotic solvents such as
benzene or
acetone in which the pathway leading to
benzoxazepines usually predominates.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
silica gel
1,3-OXAZEPINES
3,1-benzoxazepines
quinoline N-oxides
pyridine N-oxides
isoquinoline N-oxides
quinoxaline N-oxides
quinazoline N-oxides
phenanthridine N-oxides
benzophenazine N-oxides
acridine N-oxides
carbostyrils
benzoxazepines
ethanol (64-17-5)
Benzene (71-43-2)
methanol (67-56-1)
chloroform (67-66-3)
oxygen (7782-44-7)
nitrogen (7727-37-9)
mercury (7439-97-6)
cyclohexane (110-82-7)
acetone (67-64-1)
toluene (108-88-3)
Hexamethylbenzene (87-85-4)
carbostyril (59-31-4)
calcium hydride (7789-78-8)
quinoline N-oxide (1613-37-2)
isoquinoline N-oxide (1532-72-5)
chloroform-d (865-49-6)
3,1-Benzoxazepine (15123-59-8)
benzoxazepine
2-phenyl-3,1-benzoxazepine
1,3-benzoxazepine
quinoline N-oxide dihydrate
2(1H)-quinolinone
2-phenylquinoline N-oxide
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved