Org. Synth. 1983, 61, 103
DOI: 10.15227/orgsyn.061.0103
PERHYDRO-9b-BORAPHENALENE AND PERHYDRO-9b-PHENALENOL
[9b-Boraphenalene, dodecahydro- and Phenalen-9bα(2H)-ol, 3,3aα,4,5,6,6aα,7,8,9,9aβ-decahydro-]
Submitted by Ei-ichi Negishi
1,3 and Herbert C. Brown
2,3.
Checked by A. J. Cocuzza and R. E. Benson.
1. Procedure
Caution! The products used and formed in Step A are extremely pyrophoric. Great care should be taken in conducting this step.
A. cis,trans-Perhydro-9b-boraphenalene. A 1-L, three-necked, round-bottomed flask is fitted with a septum, thermometer, magnetic stirring bar, and a 12-cm Vigreux column. A 2-L, two-necked receiving flask is attached to the Vigreux column and fitted with a nitrogen-inlet tube that is attached to a mercury bubbler device to permit a positive pressure on the system (Note 1). The entire system is flushed with nitrogen and, while the system is maintained under a static pressure of nitrogen, 500 mL (0.050 mol) of a 1.0 M solution of borane in tetrahydrofuran (THF, (Note 2)) is added to the reaction flask by means of a syringe. The flask is immersed in an ice–water bath, stirring is begun, and 50.6 g (0.50 mol) of triethylamine (Note 3) is added slowly over 15 min. After the addition is completed, the THF is removed by distillation at atmospheric pressure and 300 mL of dry diglyme (Note 4) is added. The resulting solution is heated to 130–140°C and a solution of 81 g (0.50 mol) of trans,trans,trans-1,5,9-cyclododecatriene (Note 5) in 100 mL of dry diglyme (Note 6) is added over 2 hr. At the end of this time, the diglyme is removed by distillation at atmospheric pressure and the residual oil is heated at 200°C for 6 hr (Note 7). After the reaction is cooled, the thermally treated product is used directly in Step B (Note 8). Product free of polymeric impurity can be obtained by distillation (Note 9) and (Note 10).
B. cis,cis,trans-2-(Perhydro-9'b-phenalyl)-1,3,2-dioxaborole. The 250-mL pressure vessel (Note 11) is fitted with a cap bearing a rubber septum. Two hypodermic needles are inserted into the vessel through the septum, one with the end close to the bottom of the vessel and the other with the end close to the top. The vessel is flushed with nitrogen, with the exit gas passing through a mercury bubbler device. One-fifth of the thermally treated, undistilled product obtained from Step A is dissolved in 50 mL of dry THF (Note 12) and added to the vessel by means of a syringe while a static pressure of nitrogen is maintained on the vessel. Ethylene glycol (18.6 g, 16.8 mL, 0.30 mol) (Note 13) is then added. The rubber septum is removed and the vessel is quickly connected to a cylinder of carbon monoxide (Note 14) and placed in a heating device capable of agitation. The vessel is agitated and the pressure increased with carbon monoxide to ca. 70 atm (ca. 1000 psi); the temperature is raised to 150°C. The vessel is maintained at this temperature for 2 hr and then cooled to room temperature and opened to the air. The contents of the vessel are transferred to a flask; the vessel is rinsed with two 50-mL portions of pentane, and the pentane is added to the product. The resulting solution is washed with 50 mL of water and dried over magnesium sulfate. The drying agent is removed by filtration, and the pentane removed by distillation to give 19.4 g of cis,cis,trans-2-(perhydro-9'b-phenalyl)-1,3,2-dioxaborole, a solid that can be further purified by recrystallization from pentane, mp 101–102°C (Note 15).
C. cis,cis,trans-Perhydro-9b-phenalenol. A 500-mL, three-necked, round-bottomed flask is fitted with a septum, thermometer, magnetic stirring bar, and a reflux condenser, which is connected to a nitrogen inlet and a mercury bubbler device. The system is flushed with nitrogen, and 50 mL of THF, 100 mL of 95% ethanol, and 19.4 g (0.0782 mol) of cis,cis,trans-2-(perhydro-9'b-phenalyl)-1,3,2-dioxaborole from Step B are added to the flask together with 37 mL (0.220 mol, 120% excess) of 6 N sodium hydroxide. The solution is stirred and, by means of a dropping funnel, 37 mL (~0.326 mol) of 30% hydrogen peroxide (Note 16) is added at such a rate that the temperature of the reaction mixture does not exceed 40°C. After the initial reaction has subsided, the reaction mixture is heated for 2 hr at 50°C to assure complete oxidation (Note 17). At the end of this time, 300 mL of pentane is added. The mixture is transferred to a separatory funnel, and the organic layer is separated and washed three times with 50-mL portions of water and then dried over magnesium sulfate. The mixture is filtered, and the solvent is removed by distillation to yield a solid (Note 18) which is recrystallized from cold pentane to give 10.9 g (71.7%) of cis,cis,trans-perhydro-9b-phenalenol, mp 75–76°C (Note 19).
2. Notes
1.
All joints must be well greased and securely clamped. Even a minor leak is a fire hazard.
2.
The checkers used a reagent available from Aldrich Chemical Company, Inc.
Borane–
THF was prepared by the submitters.
2 The direct use of
borane–
THF for hydroboration results in the formation of a polymeric, insoluble intermediate, which can be depolymerized by heating.
3.
The checkers refluxed
triethylamine, available from Eastman Organic Chemicals, with
phenyl isocyanate and then isolated the amine by distillation. The submitters used a reagent available from Aldrich Chemical Company, Inc.
4.
The checkers used a reagent available from Aldrich Chemical Company, Inc. The
diglyme was distilled from
sodium benzophenone ketyl prior to use. The submitters used a reagent available from the same source and distilled it from
lithium aluminum hydride prior to use.
5.
The checkers and the submitters used reagent available from Chemical Samples Co. The submitters state that other isomers such as
trans,trans,cis-1,5,9-cyclododecatriene or a mixture of isomers can be used.
3 In this case a slightly different isomer distribution is observed, and the yields of isolated product are somewhat lower. The checkers confirmed this observation, using trans,trans,cis reagent available from Aldrich Chemical Company, Inc.
6.
A syringe pump was used with the syringe well greased with a polyhalo hydrocarbon lubricant. Alternatively, a
pressure-equalizing dropping funnel can be used.
7.
It is essential to heat the initially formed product to 200°C to achieve isomerization of the other isomers present to
cis,trans-perhydro-9b-boraphenalene. When this thermal treatment is omitted, the desired product is contaminated with one major (30–40%) and several minor, unidentified, isomeric substances.
8.
Perhydro-9b-boraphenalene is highly flammable. The transfer must be carried out with caution. The use of gloves is recommended to avoid direct contact with the organoborane. The transfer is most conveniently done under a slightly positive pressure of
nitrogen using a broad-gauge (18-gauge), double-tipped needle.
9.
The crude product is diluted with a small amount of dry
THF and the solution transferred to a
100-mL distillation flask. Distillation through a 12-cm Vigreux column gives 58.0–60.1 g (66–68% yield) of a mixture of
cis,trans- and cis,cis-perhydro-9b-boraphenalene, bp
113–114°C (9.5 mm),
1H NMR (CDCl
3) δ: 0.7–2.2.
10.
The submitters state that the composition of the distillate is 92 : 8 cis,trans : cis,cis isomer, based on GC analyses using an SE-30 column. The assigned stereochemistry is supported by the
1H NMR spectrum of the pyridine complex.
311.
The checkers used a
250-mL Hastelloy pressure vessel. The submitters used a
250-mL autoclave available from American Instrument Co.
12.
The checkers used a reagent available from Fisher Scientific Company. The submitters used a reagent available from Aldrich Chemical Company, Inc.
13.
The checkers distilled the reagent available from E. I. du Pont de Nemours & Co. The submitters used a reagent available from Aldrich Chemical Company, Inc.
14.
The checkers and submitters used a reagent available from Matheson Gas Products.
15.
The checkers obtained the product in
87% crude yield using distilled
boraphenalene. Recrystallization from
pentane gave product in
66% yield, mp
101–102°C, with the following spectral characteristics: IR (KBr) cm
−1: 1185, 1200, 1250, 1310, 1385, 2860, and 2900–2950;
1H NMR (CDCl
3) δ: 1.0–1.9 (m, 21 H), 4.11 (s, 4 H). The structure of the product has been confirmed by X-ray crystallography.
316.
The checkers and the submitters used reagent available from Fisher Scientific Company.
17.
The submitters state that oxidation of the dioxaborole is unusually sluggish and urge the use of
ethanol as a cosolvent and an excess of 6
N sodium hydroxide. They also urge monitoring of the reaction by GC. The checkers monitored the reaction by both GC and TLC analyses. GC analysis by the checkers was conducted using the following column and conditions: 3.2-mm × 2-m column, 7% SE 30/3% Silar on Gas Chrom Q (60–80 mesh), 170°C,
50 mL of nitrogen per min. The retention times for the
perhydrophenalenol and dioxaborole are 6.4 and 15.2 min, respectively. For TLC analyses, Analtech silica gel plates bearing the material were eluted by 1 : 2
methylene chloride–petroleum ether, and visualized with
phosphomolybdic acid:
perhydrophenalenol,
Rf 0.4; dioxaborole,
Rf 0.9. The checkers found that the reaction was essentially complete after addition of the
hydrogen peroxide. Additional heating did not lower the yield of product.
18.
The submitters state that GC analysis of the solid indicates a 92 : 8 mixture of the cis,cis,trans and cis,cis,cis isomers.
19.
Using recrystallized dioxaborole from Step B, the checkers obtained product, recrystallized from
pentane, mp
75–76°C, in
85% yield, having the following spectral characteristics: IR (KBr) cm
−1: 1450 (s), 2860 (m), 2930 (s), and 3460 (m);
1H NMR (CDCl
3) δ: 1.0–2.3;
13C NMR (CDCl
3) δ: 21.25 (t), 26.52 (t), 27.42 (t), 29.79 (t), 29.12 (t), 33.86 (d), 44.32 (d), 73.24 (s) (undecoupled spectrum).
3. Discussion
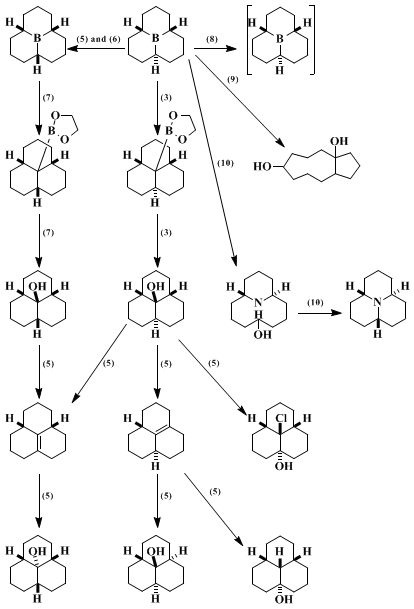
Preparation of the two stereoisomers of
perhydro-9b-boraphenalene was originally reported by Köster and Rotermund,
5 and the present procedure (Step A) is largely based on the procedure described by these authors. However, the original stereochemical assignment was incorrect and has been reversed.
3,6 Furthermore, these authors did not use the thermal treatment described above, which appears essential to achieve isomerization of other constitutional isomers into
perhydro-9b-boraphenalene.
3 The original procedure for isomerization of the cis,trans isomer to the all cis isomer has been satisfactory. Contrary to the claim made by these authors,
5 however, this isomerization does not lead quantitatively to the all cis isomer, but reaches an equilibrium, which consists of the all cis and cis,trans isomers in the ratio of 88 : 12; this ratio was also confirmed by reverse isomerization of the pure all cis isomer.
7cis,trans-Perhydro-9b-boraphenalene has been converted to
lithium cis,cis,trans-perhydro-9b-boraphenalyl hydride by reaction with
lithium hydride.
8 The tricyclic organoborane reported here has been converted to
bicyclo[7.3.1]dodecane-1,5-diol9 and
trans-13-azabicyclo[7.3.1]tridecan-5-ol.
10 The latter has been converted to
cis,trans-perhydro-9b-azaphenalene.
10
The procedure reported here (Steps B and C) has been applied with minor modifications to the syntheses of the cis,cis,cis isomers of the 1,3,2-dioxaborole and
perhydro-9b-phenalenol.
6 The two other stereoisomers, cis,trans,trans and trans,trans,trans, have been prepared from
cis,cis,trans-perhydro-9b-phenalenol via the cis and trans isomers of Δ
3a,
9b-perhydrophenalene.
7 In addition, a few isomers of
perhydrophenalenol and of perhydrophenalene and
cis,cis,trans-9b-chloroperhydrophenalene have also been prepared from
cis,cis,trans-perhydro-9b-phenalenol.
7 Some of the representative transformations are summarized in Scheme 1. (The numbers in parentheses refer to references.)
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
petroleum ether
sodium benzophenone ketyl
cis,trans- and cis,cis-perhydro-9b-boraphenalene
lithium cis,cis,trans-perhydro-9b-boraphenalyl hydride
bicyclo[7.3.1]dodecane-1,5-diol
ethanol (64-17-5)
sodium hydroxide (1310-73-2)
carbon monoxide (630-08-0)
nitrogen (7727-37-9)
ethylene glycol (107-21-1)
hydrogen peroxide (7722-84-1)
Pentane (109-66-0)
methylene chloride (75-09-2)
phenyl isocyanate (103-71-9)
magnesium sulfate (7487-88-9)
borane (7440-42-8)
Tetrahydrofuran,
THF (109-99-9)
lithium aluminum hydride (16853-85-3)
triethylamine (121-44-8)
diglyme (111-96-6)
lithium hydride (7580-67-8)
phosphomolybdic acid (51429-74-4)
Perhydro-9b-boraphenalene,
9b-Boraphenalene, dodecahydro-,
cis,trans-perhydro-9b-boraphenalene (16664-33-8)
Perhydro-9b-phenalenol,
Phenalen-9bα(2H)-ol, 3,3aα,4,5,6,6aα,7,8,9,9aβ-decahydro- (16664-34-9)
boraphenalene
perhydrophenalenol
trans,trans,cis-1,5,9-cyclododecatriene
trans,trans,trans-1,5,9-cyclododecatriene (676-22-2)
cis,cis,trans-perhydro-9b-phenalenol
trans-13-azabicyclo[7.3.1]tridecan-5-ol
cis,trans-perhydro-9b-azaphenalene
cis,cis,trans-9b-chloroperhydrophenalene
cis,cis,trans-2-(perhydro-9'b-phenalyl)-1,3,2-dioxaborole
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved