Org. Synth. 1987, 65, 236
DOI: 10.15227/orgsyn.065.0236
1-DEOXY-2,3,4,6-TETRA-O-ACETYL-1-(2-CYANOETHYL)-α-D-GLUCOPYRANOSE
[D-glycero-D-ido-Nonononitrile, 4,8-anhydro-2,3-dideoxy-, 5,6,7,9-tetraacetate]
Submitted by Bernd Giese, J. Dupuis, and Marianne Nix
1.
Checked by John P. Daub and Bruce E. Smart.
1. Procedure
Caution! Acrylonitrile is an OSHA-regulated carcinogen and appropriate precautions should be taken in handling this substance.
A 250-mL, three-necked, round-bottomed flask equipped with a magnetic stirring bar, a dry-nitrogen inlet, a reflux condenser, and a septum is flushed with nitrogen and charged with 20.6 g (50 mmol) of 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (Note 1) and 100 mL of anhydrous ether. The mixture is brought to reflux with a heat gun. A nitrogen atmosphere is maintained over the reaction mixture during this and the ensuing steps. When the 2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide dissolves, 13.5 g (250 mmol) of acrylonitrile and 16.0 g (55 mmol) of tributyltin hydride (Note 2) are added by means of a syringe. The refluxing solution is irradiated with a sunlamp positioned 2.5–3.8 cm from the reaction flask (Note 3). The heat from the sunlamp maintains a vigorous reflux. After 4 hr, the irradiation is stopped and the reaction mixture is cooled and filtered to collect the precipitated crystals (Note 4). The filtrate is reintroduced under nitrogen into the reaction vessel. An additional 6.6 g (120 mmol) of acrylonitrile and 5.8 g (20 mmol) of tributyltin hydride are added and irradiation is resumed for another 4 hr until the starting bromide is completely reacted (Note 5). After the reaction mixture is cooled, it is filtered, the filtrate is saved, and the filter cake (Note 6) is combined with the crystals collected above. The solids are stirred with 250 mL of hot isopropyl alcohol and filtered. The hot filtrate is concentrated to a total volume of 75 mL, allowed to cool and filtered to give 7.8–8.6 g (40–45%) of 1-deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-α-D-glucopyranose as long, colorless crystals, mp 121–122°C, [α]D25 +66.2 (CHCl3, c 0.7) (Note 7). The isopropyl alcohol mother liquor is set aside. The above filtrate from the crude reaction mixture is concentrated, taken up in 50 mL of acetonitrile, and extracted 3 times with 50-mL portions of pentane. The acetonitrile phase is combined with the isopropyl alcohol mother liquor and concentrated. The resulting syrup is flash-chromatographed on silica gel using ethyl acetate/hexane (1 : 1) eluent to afford an additional 2.2–2.5 g of pure 1-deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-α-D-glucopyranose after separation of by-products (Note 8) and (Note 9). The combined yield of pure product is 53–56%
2. Notes
1.
This material was obtained from the Sigma Chemical Company and was recrystallized from
ethyl ether/
pentane before use. It also can be prepared by the procedure of Redemann, C. E.; Niemann, C.
Org. Synth., Coll. Vol. III, 1955, 11.
2.
Acrylonitrile and tributyltin hydride were obtained from the Aldrich Chemical Company, Inc. and used without further purification. The use of a large excess of
acrylonitrile reduced the amount of reduction by-product
(2,3,4,6-tetraacetyl-1,5-anhydroglucitol). Because
tributyltin hydride also reacts with
acrylonitrile, a small excess must be used.
3.
The checkers used a 275-W General Electric sunlamp. The submitters indicate that the type of sunlamp is not critical and that a 125-W Philips E99/2 sunlamp or a 125-W Philips 57203 high-pressure mercury lamp are suitable. If the heat from the sunlamp does not maintain reflux, the reaction flask should be about one-fourth submerged into a hot
oil bath.
4.
During the reaction the amount of precipitate gradually increases until the light from the sunlamp is totally blocked and the reaction stops.
5.
The reaction can be followed by TLC using
0.25-mm silica gel 60 F-254 plates (E. Merck & Company) and
ethyl acetate/
hexane (1 : 1) eluent:
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide (
Rf = 0.39);
2,3,4,6-tetra-O-acetyl-1,5-anhydroglucitol (
Rf = 0.28);
1-deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-β-D-glucopyranose (
Rf = 0.20);
1-deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-α-D-glucopyranose (
Rf = 0.16).
6.
This solid contains a large amount of insoluble polymer in addition to product.
7.
The product is analytically pure. Anal. calcd. for C
17H
23NO
9: C, 52.98; H, 6.02; N, 3.63. Found: C, 52.80; H, 6.01; N, 3.63;
1H NMR (CDCl
3, 360 MHz): 1.84–1.95 (m, 1 H, CCH
2), 2.05 (s, 6 H, 2 OAc), 2.09 (s, 3 H, OAc), 2.11 (s, 3 H, OAc), 2.05–2.22 (m, 1 H, CCH
2), 2.46 (m, 2 H, CH
2CN), 3.88 (ddd, 1 H, H
5,
J5,6 = 5.80,
J5,6' = 2.88,
J4,5 = 8.30), 4.12 (dd, 1 H, H
6',
J6,6' = 12.24), 4.23 (m, 1 H, H
1), 4.32 (dd, 1 H, H
6), 4.98 (t, 1 H, H
4,
J3,4 = 8.30), 5.09 (dd, 1 H, H
2,
J1,2 = 5.23,
J2,3 = 8.30), 5.23 (t, 1 H, H
3); IR (KBr) cm
−1: 2240 (C≡N), 1745 (C=O).
8.
E.
Merck 230–400 mesh Kieselgel 60 silica gel was used in the flash chromatography. The first fraction contained
3.5 g (
21%) of the
tetra-O-acetyl-1,5-anhydroglucitol by-product; the second fraction contained
0.95–1.00 g (
5%) of pure
1-deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-β-D-glucopyranose: mp
91–93°C, 117–119°C (two forms);
[α]D25 −19.6 (CHCl
3,
c 1.01). The final fraction contained the desired product. The ratio of α : β isomers based on isolated yields is 11 : 1. The submitters report a ratio of 14 : 1 based on chromatographic analysis of the crude reaction mixture.
9.
1-Deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-β-D-glucopyranose shows IR (KBr) cm
−1: 2240 (C≡N), 1745 (C=O);
1H NMR (CDCl
3, 360 MHz) δ: 1.79 (br m, 1 H, CCH
2), 1.92 (br m, 1 H, CCH
2), 2.00 (s, 3 H, OAc), 2.03 (s, 3 H, OAc), 2.07 (s, 3 H, OAc), 2.09 (s, 3 H, OAc), 2.52 (m, 2 H, CH
2CN), 3.56 (dt, 1 H, H
1,
J1,2 = 9.6,
J = 9.6, 2.6), 3.68 (ddd, 1 H, H
5,
J4,5 = 9.9,
J5,6 = 5.0,
J5,6' = 2.1), 4.11 (dd, 1 H, H
6',
J6,6' = 12.2), 4.24 (dd, 1 H, H
6), 4.88 (t, 1 H, H
2,
J2,3 = 9.6), 5.05 (t, 1 H, H
4,
J3,4 = 9.4), 5.20 (t, 1 H, H
3). Anal. calcd. for C
17H
23NO
9: C, 52.98; H, 6.02; N, 3.63. Found: C, 52.85; H, 6.09; N, 3.70.
3. Discussion
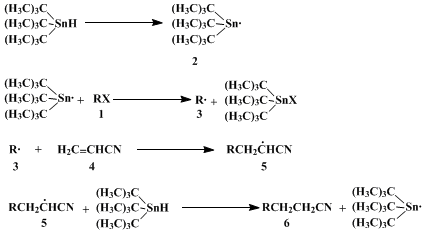
The formation of the C-C bond occurs in a radical chain reaction
2 (Scheme 1).
Bromine abstraction from halide
1 by
tin radicals
23 leads to
carbon radicals
3 that react with alkenes
4 to give product radicals
5. Trapping of
5 by
tributyltin hydride yields products
6 and the
tin radical
2.
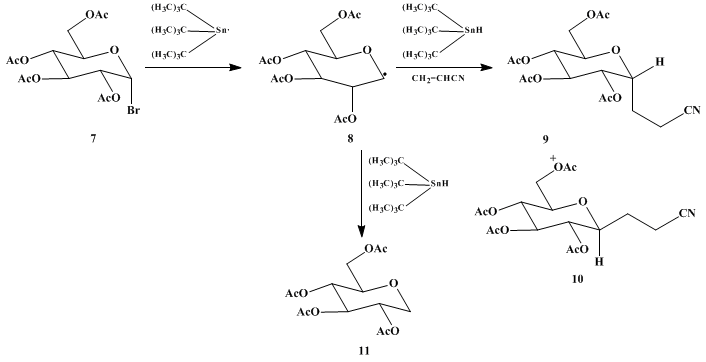
In contrast to anionic 1,4-addition methods, the radical procedure tolerates an acetoxy group adjacent to the reactive center (radical-bearing
carbon). In applying this general method to
tetraacetylglucosyl bromide 7,
4 products
9, with an axial C-C bond, and
10 are obtained in a ratio of ca. 10 : 1. ESR experiments show that the radical intermediate has the boat conformation
8.
5 The attack that leads to the axial product
9 is, therefore, the favored pseudoequatorial attack,
6 assuming that the transition state is also boatlike
8. Some of the radical
8 is trapped directly by
tributyltin hydride to give the simple reduction product
11. This is a common side reaction in
tin hydride-initiated radical coupling reactions.
This method has been applied also to
mannosyl bromide and
galactosyl bromide.
4 Because alkoxyalkyl radicals are nucleophilic radicals, only alkenes with electron-withdrawing substituents can be used.
7 The 1,5-anhydroglycitol side product
11 is formed in amounts that increase with the decreasing reactivity of alkene
4.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
1-Deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-α-D-glucopyranose
(2,3,4,6-tetraacetyl-1,5-anhydroglucitol)
2,3,4,6-tetra-O-acetyl-1,5-anhydroglucitol
1-deoxy-2,3,4,6-tetra-O-acetyl-1-(2-cyanoethyl)-β-D-glucopyranose
tetra-O-acetyl-1,5-anhydroglucitol
mannosyl bromide
D-glycero-D-ido-Nonononitrile, 4,8-anhydro-2,3-dideoxy-, 5,6,7,9-tetraacetate
ethyl acetate (141-78-6)
ether,
ethyl ether (60-29-7)
acetonitrile (75-05-8)
bromine (7726-95-6)
nitrogen (7727-37-9)
tin (7440-31-5)
carbon (7782-42-5)
isopropyl alcohol (67-63-0)
Pentane (109-66-0)
acrylonitrile (107-13-1)
hexane (110-54-3)
tributyltin hydride (688-73-3)
tetraacetylglucosyl bromide
galactosyl bromide
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved