Org. Synth. 1987, 65, 32
DOI: 10.15227/orgsyn.065.0032
PREPARATION AND THREE-CARBON + TWO-CARBON CYCLOADDITION OF CYCLOPROPENONE 1,3-PROPANEDIOL KETAL: 5,5-DICYANO-4-PHENYL-2-CYCLOPENTEN-1-ONE 1,3-PROPANEDIOL KETAL
[4,8-Dioxaspiro[2.5]oct-1-ene and 6,10-Dioxaspiro[4.5]dec-3-ene-1,1-dicarbonitrile, 2-phenyl-]
Submitted by Dale L. Boger, Christine E. Brotherton, and Gunda I. Georg
1.
Checked by Steven K. Davidsen Clayton and H. Heathcock.
1. Procedure
A.
2-(Bromomethyl)-2-(chloromethyl)-1,3-dioxane.
2 A
100-mL, round-bottomed flask is equipped with a 10-mL Dean–Stark apparatus and a condenser. The flask is charged with
30.0 g (0.138 mol) of 1-bromo-3-chloro-2,2-dimethoxypropane (Note 1),
10.0 mL (0.138 mol) of 1,3-propanediol (Note 2), and 3 drops of concentrated
sulfuric acid. The resulting solution is heated (bath temperature 140°C) for 8 hr
(Note 3) with distillative removal of
methanol (ca. 11 mL). The mixture is allowed to cool to room temperature and the crude product is partitioned in
150 mL of pentane and 40 mL of water. The organic phase is dried with
magnesium sulfate and the solvent is removed under reduced pressure
(Note 4). Distillation (
1 mm, 90–95°C) yields
25.5–27.7 g (
81–88%) of
2-(bromomethyl)-2-(chloromethyl)-1,3-dioxane, mp
57–59°C (Note 5).
B.
Cyclopropenone 1,3-propanediol ketal.
3 A
1000-mL, three-necked, round-bottomed flask is equipped with a
gas inlet, an
acetone–dry ice condenser with a
drying tube containing potassium hydroxide pellets, a
stopper, and a
magnetic stirring bar. The flask is placed in an
acetone–dry ice bath and anhydrous
ammonia (Note 6) is condensed into the flask (400 mL). A small piece of
potassium metal (ca. 0.5 g) is added to the liquid ammonia and the cooling bath is removed. A catalytic amount of anhydrous
ferric chloride (0.1 g) is added and the reaction mixture is allowed to warm to reflux temperature, at which time the deep blue color turns to gray. The remaining
potassium metal (12.2 g, 0.31 g-atom total) is added in 0.5-g pieces over ca. 30 min. The reaction mixture is allowed to stir until a gray suspension results (20–30 min). A −50°C cooling bath is placed under the flask and the stopper is replaced with a
125-mL, pressure-equalized dropping funnel containing
22.9 g (0.1 mol) of 2-(bromomethyl)-2-(chloromethyl)-1,3-dioxane in
50 mL of anhydrous ether. This solution is added dropwise to the solution of freshly generated
potassium amide over 15 min while the temperature is maintained at −50°C
(Note 7). After the solution is stirred for 3 hr at −50° to −60°C, solid
ammonium chloride is added slowly to quench the excess
potassium amide (Note 8). The cooling bath is removed and the
ammonia is allowed to evaporate. During the course of the evaporation, anhydrous
ether (350 mL total) is added dropwise through the addition funnel to replace the
ammonia. After the temperature has reached 0°C, the brown reaction mixture is filtered by suction through a coarse
fritted-glass filter to remove the inorganic salts and the salts are washed twice with
25 mL of anhydrous ether. The combined ethereal filtrate and washes are concentrated under reduced pressure (80–100 mm, 30°C) to constant weight (ca. 4–5 hr)
(Note 9). The residue is transferred to a
50-mL, round-bottomed flask fitted with a
water-cooled, short-path distillation head, and the product is distilled (
1.25 mm; 30–35°C) into an
ice-cooled receiver.
Cyclopropenone 1,3-propanediol ketal is obtained as a colorless liquid (
6.1–7.8 g,
55–70% yield,
(Note 10)).
C. 5,5-Dicyano-4-phenyl-2-cyclopenten-1-one 1,3-propanediol ketal. Benzylidenemalononitrile (Note 2) (3.85 g, 25 mmol) and cyclopropenone 1,3-propanediol ketal (5.6 g, mmol) are combined in 25 mL of dry distilled toluene (Note 11) in a sealed tube (Note 12) with a magnetic stirring bar. The reaction mixture is heated at 80°C for a 6.5 hr with stirring. The crude reaction mixture is filtered through a short plug of glass wool and applied to a Waters Associates Prep LC/System 500, eluting with 2.5 : 1 hexane/ethyl acetate (Note 13). The fractions containing product are combined and concentrated under reduced pressure to give 4–4.2 g (60–64%) of 5,5-dicyano-4-phenyl-2-cyclopenten-1-one 1,3-propanediol ketal as a white solid: mp 139–141°C (ethanol, (Note 14)).
2. Notes
2.
The submitters employed material available from Aldrich Chemical Company, Inc. without further purification.
3.
Shorter reaction times result in incomplete transketalization.
4.
The checkers found that the product crystallizes, either on removal of solvent or in the condenser during the following distillation. To prevent the condensers tube becoming plugged by the crystalline product, an
air-cooled, rather than a water-cooled, condenser jacket should be used.
5.
The product has the following spectral properties:
1H NMR (CDCl
3) δ: 1.78 (m, 2 H, CH
2), 3.70 (s, 2 H, CH
2Cl), 3.80 (s, 2 H, CH
2Br), 3.96 (t, 4 H,
J = 6, OCH
2); IR (CHCl
3) cm
−1: 3030, 3000, 2900, 1485, 1430, 1245, 1202, 1158, 1135, 1105 (s), 1020.
6.
Commercial anhydrous
ammonia is employed without further drying.
7.
Crystals that may form at the tip of the addition funnel are scraped off and allowed to drop into the reaction flask.
8.
The checkers added a total of
25 g of solid ammonium chloride in portions of approximately 2 g with a
spatula.
9.
The checkers removed the solvent with a
rotary evaporator at 20 mm and 25°C; under these conditions only 1 hr is required to concentrate the solution to a constant weight.
10.
This material should be stored under an
argon atmosphere below 0°C. The product has the following spectral properties:
1H NMR (CDCl
3) δ: 1.83 (m, 2 H, CH
2), 4.01 (t, 4 H,
J = 6, OCH
2), 7.84 (s, 2 H, CH=CH);
13C NMR (CDCl
3) δ: 25.4 (CH
2), 65.6 (OCH
2), 80.5 (OCO), 125.1 (C=C); IR (film) cm
−1: 3101, 2980, 2870, 1600, 1475, 1460, 1435, 1370, 1300, 1275, 1155, 1090, 1030, 935, 910, 865, 740.
11.
Toluene was distilled from
calcium hydride under a
nitrogen atmosphere.
12.
The resealable glass tube was fabricated from an Ace Glass medium-walled straight tube. The tube was permanently sealed on one end, and the other end remained internally threaded. The tube was sealed with a solid Teflon plug fitted with a FETFE O-ring. Various sizes of such tubes are now available from Ace Glass.
13.
The submitters used medium pressure chromatography
5 on
25 × 1000 mm of 230–400 mesh silica gel with a 25 × 250-mm scrubber column. The eluant (
3 : 1 : 1 hexane : ethyl acetate: methylene chloride) was passed through the column at a rate of 15 mL/min, collecting 15-mL fractions. The checkers were unable to obtain pure product using a gravity column or flash chromatography.
14.
The product has the following spectral properties:
1H NMR (CDCl
3) δ: 1.71 (dt, 1 H,
J = 13.8,
J = 3.8, OCH
2CH
2CH
2O), 2.1–2.3 (broad m, 1 H, OCH
2CH
2CH
2O), 3.9–4.3 (m, 4 H, OCH
2CH
2CH
2O), 4.63 (t, 1 H,
J = 2.3, allylic CH), 6.25 (dd, 1 H,
J = 6.3,
J = 2.0, CH-CH=CH-C), 6.62 (dd, 1 H,
J = 6.3,
J = 2.6, CH-CH=CH-C), 7.3–7.5 (m, 5 H, phenyl); IR (CHCl
3) cm
−1: 3070, 2920, 2290 (C≡N), 1510, 1465, 1350, 1260, 1210, 1175, 1140, 1100, 1080, 1040, 870, 705.
3. Discussion
Although a number of multistep procedures are available for the introduction of five-membered carbocycles, their direct formation in a thermal cycloaddition is rare.
6 Interest in the potential application of such a three-carbon + two-carbon
cyclopentane cycloaddition has been derived from the expectation that such a process could prove to be an effective complement to the four-carbon + two-carbon Diels–Alder reaction, which is used extensively in the regio- and stereocontrolled preparation of functionalized six-membered carbocycles.
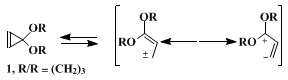
Cyclopropenone ketals, of which
cyclopropenone 1,3-propanediol ketal (
1) is a representative and unusually stable example, have proved to be useful equivalents of the 1,3-dipole (
i) in a regiospecific three-carbon + two-carbon cycloaddition with electron-deficient olefins (Eq. 1). Table I shows representative results of a study of this reaction.
7
TABLE I
REACTIONS OF CYCLOPROPENONE KETAL 1 WITH ELECTRON-DEFICIENT OLEFINS
|
|
Substrate
|
Conditionsa equiv 1, temp. °C(time hr)
|
Product
|
%Yield
|
|
|
|
1.0–2.0, 80 (12)
|
|
42%
|
|
|
1.0, 75 (13)
|
|
45%
|
|
|
R = Et 1.5, 75 (13)
|
|
53%
|
R = Me 2.5, 75 (32)
|
60%
|
|
|
1.5, 75 (15)
|
|
57%
|
|
|
2.0, 80 (5)
|
|
84%
|
|
|
2.0, 80 (4)
|
|
60–64%
|
|
a All reactions were run in benzene (0.5–2.0 M in substrate) under nitrogen unless otherwise noted.
|
Studies have defined the scope of the thermal reactions of cyclopropenone ketals which are characterized by their thermal, reversible ring opening to provide reactive intermediates best represented as delocalized singlet vinyl carbenes, three-carbon 1,1-/1,3-dipoles without octet stabilization (Eq. 2).
In addition to the representative [3 + 2] cycloaddition reactions shown in Table I, the delocalized singlet vinyl carbenes have been shown to participate as π2
a components of nonlinear cheletropic [π2
s + π2
a] cycloadditions to provide cyclopropanes with an observable endo effect,
8 and as π2
s components of [π4
s + π2
s] cycloadditions with selected dienes to provide cycloheptadienes
9,10 (Scheme 1). This thermal reactivity of cyclopropenone ketals complements their dual participation as strained olefins in normal (HOMO
diene controlled) and inverse electron demand (LUMO
diene controlled) Diels–Alder reactions with electron-rich, electron-deficient, and neutral dienes under room temperature, thermal, and pressure-promoted Diels–Alder conditions.
11,12,13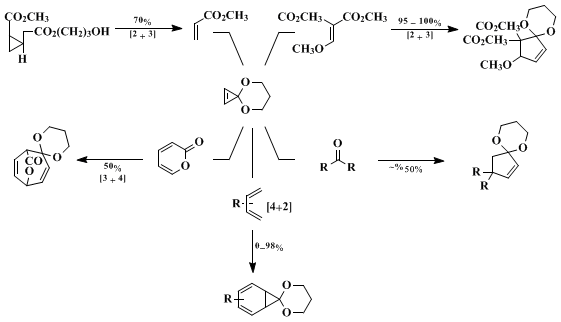
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Cyclopropenone 1,3-propanediol ketal
5,5-Dicyano-4-phenyl-2-cyclopenten-1-one 1,3-propanediol ketal
ethanol (64-17-5)
sulfuric acid (7664-93-9)
ammonia (7664-41-7)
Benzene (71-43-2)
ethyl acetate (141-78-6)
methanol (67-56-1)
ether (60-29-7)
ammonium chloride (12125-02-9)
1,3-propanediol (504-63-2)
nitrogen (7727-37-9)
toluene (108-88-3)
ferric chloride (7705-08-0)
potassium (7440-09-7)
Pentane (109-66-0)
methylene chloride (75-09-2)
magnesium sulfate (7487-88-9)
potassium amide
hexane (110-54-3)
argon (7440-37-1)
1-Bromo-3-chloro-2,2-dimethoxypropane (22089-54-9)
calcium hydride (7789-78-8)
cyclopentane (287-92-3)
4,8-Dioxaspiro[2.5]oct-1-ene (60935-21-9)
6,10-Dioxaspiro[4.5]dec-3-ene-1,1-dicarbonitrile, 2-phenyl- (88442-12-0)
2-(Bromomethyl)-2-(chloromethyl)-1,3-dioxane (60935-30-0)
Benzylidenemalononitrile (2700-22-3)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved