Org. Synth. 1989, 67, 149
DOI: 10.15227/orgsyn.067.0149
ETHYNYL p-TOLYL SULFONE
[Benzene, 1-(ethynylsulfonyl)-4-methyl-]
Submitted by Liladhar Waykole and Leo A. Paquette
1.
Checked by Dirk A. Heerding and Larry E. Overman.
1. Procedure
A. p-Tolyl 2-(trimethylsilyl)ethynyl sulfone. In a flame-dried, 500-mL, three-necked, round-bottomed flask fitted with a nitrogen inlet and glass stoppers are placed 200 mL of dry dichloromethane (Note 1) and 29.4 g (0.22 mol) of freshly powdered anhydrous aluminum chloride. After the addition of p-toluenesulfonyl chloride (41.9 g, 0.22 mol), the resulting dark-brown mixture is shaken occasionally for 20 min at room temperature.
A 1-L, three-necked, round-bottomed flask equipped with a 500-mL addition funnel and a Teflon-coated stirring bar is flame-dried under a stream of dry nitrogen. The flask is charged with bis(trimethylsilyl)acetylene (34.0 g, 0.20 mol) (Note 2) and dry dichloromethane (200 mL) (Note 1) and the solution is cooled to 0°C in an ice–water bath.
The p-toluenesulfonyl chloride–aluminum chloride complex is quickly filtered through a glass wool plug (Note 3) into the addition funnel. The residue is washed rapidly with an additional 50 mL of dry dichloromethane and the funnel is quickly stoppered. The complex is added dropwise during 1 hr to the cold (0°C), magnetically stirred silylacetylene solution. On completion of the addition, the reaction mixture is allowed to warm to room temperature and is stirred for an additional 12 hr. The mixture is hydrolyzed by pouring it into a slurry of 20% hydrochloric acid (200 mL) and ice (200 g) (Note 4). The organic layer is separated, washed twice with water (150 mL), and dried over anhydrous sodium sulfate. Removal of solvent in a rotary evaporator gives a brown solid (Note 5) that is recrystallized from light petroleum ether (bp 40–60°C) to yield 39.7–40.4 g (79–80%) of p-tolyl 2-(trimethylsilyl)ethynyl sulfone as white crystals, mp 81–82°C (Note 6).
B. Ethynyl p-tolyl sulfone. A 1-L, three-necked, round-bottomed flask equipped with a thermometer, a 500-mL addition funnel, a nitrogen inlet, and a Teflon-coated magnetic stirring bar is charged with p-tolyl 2-(trimethylsilyl)ethynyl sulfone (25.2 g, 0.1 mol) and 300 mL of reagent-grade methanol. After the mixture is stirred for 30 min, a clear solution is obtained. In the addition funnel is placed 350 mL of an aqueous solution containing potassium carbonate (6.2 × 10−3 M) and potassium bicarbonate (6.2 × 10−3 M); this buffer is added at a rate to maintain the reaction temperature at 30°C (Note 7) and (Note 8). The mixture is diluted with water (200 mL), and extracted with four 100-mL portions of chloroform. The combined organic phases are washed 3 times with water (100 mL) and twice with brine (100 mL) prior to drying over anhydrous sodium sulfate. Removal of solvent under reduced pressure leaves a creamy white solid, which is purified by either recrystallization from ethyl acetate–petroleum ether or silica gel chromatography using 10% ethyl acetate in petroleum ether as eluant (Note 9). There is obtained 15.0 g (83%) of colorless crystals, mp 74–75°C (Note 10) and (Note 11).
2. Notes
1.
The submitters used
dichloromethane freshly distilled from powdered
calcium hydride.
2.
This reagent was obtained from Petrarch Systems, Inc., Bartram Road, Bristol, PA 19007.
3.
This mixture is rather hygroscopic and must be maintained under a
nitrogen atmosphere as much as possible.
4.
Stirring facilitates the hydrolysis. The reaction mixture should be added relatively slowly since the decomposition is exothermic.
5.
Material of this purity may be used directly in the ensuing step. However, lower yields are realized.
6.
Earlier citations
2,3 report mp
81–82°C. This product has the following spectral properties: IR (KBr) cm
−1: 2124, 1338, 1164, 854, 779;
1H NMR (CDCl
3) δ: 0.22 (s, 9 H), 2.48 (s, 3 H), 7.40 (d, 2 H,
J = 9), 7.91 (d, 2 H,
J = 9). MS (CI, 70 eV, isobutane) 253 (M
+ + 1, 100). Anal. calcd. for C
12H
16O
2SSi: C, 57.10; H, 6.40; S, 12.70. Found: C, 57.84; H, 5.88; S, 12.85. The checkers found that treatment of the crude solid with activated charcoal is required to obtain colorless product.
7.
The checkers found that the reaction is complete immediately after addition if the temperature is maintained accurately at 30°C. The reaction rate is dramatically dependent on the reaction temperature. The submitters report that considerable resinous material is obtained if the temperature goes above 30°C.
8.
This period of reaction may vary depending on the scale of the reaction. Progress may be easily followed by isolating aliquots and obtaining
1H NMR spectra. The disappearance of the
trimethylsilyl singlet is the observable diagnostic.
9.
The checkers found that an impurity with a characteristic
1H NMR singlet at 3.74 ppm is readily removed by recrystallization, but cannot be removed by chromatography. They also report that small amounts of this impurity are formed during flash chromatography on silica gel.
10.
The
1H NMR spectral characteristics of this sulfone are as follows (CDCl
3) δ: 2.47 (s, 3 H), 3.52 (s, 1 H), 7.38 (dd, 2 H,
J = 8.5, 0.6), 7.88 (d, 2 H,
J = 8.5). Its IR spectrum (KBr) consists of the following bands (cm
−1): 3235, 2013, 1337, 1156. MS (CI, 70 eV, isobutane) 181 (M
+ + 1, 100). Anal. calcd. for C
9H
8SO
2: C, 59.97; H, 4.47; S, 17.79. Found: C, 59.20; H, 4.55; S, 17.52.
11.
Further purification can be achieved if desired by recrystallization of this material from
hexane–
ethyl acetate (95 : 5). Shiny needles that melt at 75°C are thereby obtained.
3. Discussion
Interest in arylsulfonyl acetylenes arose initially because of their powerful Michael acceptor properties. Examples of facile nucleophilic addition involving thiolates (Eq. 1),
4,5,6,7 amines,
2 cuprates (Eq. 2),
8 malonate anions,
9 alkoxides,
10,11 hydroxylamines,
12,13 and azlactone enolates
14 abound. More recently, the dienophilic properties of this class of compounds have been used to advantage, such as use of the title compound as an
acetylene synthon in Diels–Alder cycloadditions,
15,16 its [4+2] capture by
N-methoxycarbonylpyrrole in a first step toward the elusive
7-azanorbornadiene,
17 and its pivotal role in a synthesis of [4]-peristylane (Eq. 3).
18 Ethynyl p-tolyl sulfone undergoes EtAlCl
2-catalyzed ene reactions with alkenes to give 1,4-dienyl
p-tolyl sulfones (Eq. 4).
19 Condensations with ynamines to give 2-amino-5-arylsulfinylfurans (Eq. 5) have been reported.
20 α,β-Acetylenic sulfones also react with organolithium and Grignard reagents to give the correspondingly higher
acetylene (Eq. 6).
21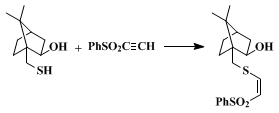
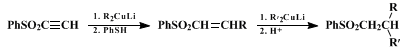
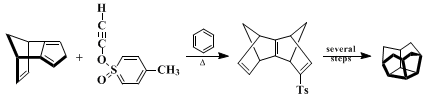
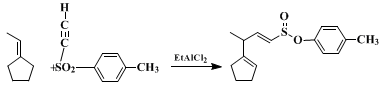
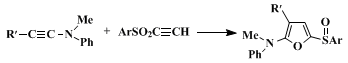
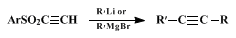
The procedures used most often for preparation of arylsulfonyl acetylenes involve oxidation of the corresponding ethynyl thio ether. The thio ethers are usually obtained via a two-step sequence beginning with twofold thiophenoxide displacement of chloride ion from
cis-1,2-dichloroethylene, followed by elimination with
n-butyllithium in the resultant
cis-1,2-bisarylthioethylene.
19 Less well known methods involve diazotization of 4-arylsulfonyl-5-aminoisoxazoles,
22 dehydrobromination of
cis- and
trans-2-bromovinyl phenyl sulfone with fluoride ion,
23 and oxidative elimination of β-(phenylseleno)vinyl sulfones.
24 The method described here, which bypasses the need for strongly basic conditions, is adapted from the work of Bhattacharya et al.
3 The simplicity and mildness of the method suggest that it may be broadly useful.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
petroleum ether
brine
potassium carbonate (584-08-7)
acetylene (74-86-2)
hydrochloric acid (7647-01-0)
ethyl acetate (141-78-6)
methanol (67-56-1)
chloroform (67-66-3)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
aluminum chloride (3495-54-3)
dichloromethane (75-09-2)
n-butyllithium (109-72-8)
hexane (110-54-3)
potassium bicarbonate (298-14-6)
calcium hydride (7789-78-8)
trimethylsilyl (16571-41-8)
p-Toluenesulfonyl chloride (98-59-9)
bis(trimethylsilyl)acetylene (14630-40-1)
Benzene, 1-(ethynylsulfonyl)-4-methyl-,
ETHYNYL p-TOLYL SULFONE (13894-21-8)
silylacetylene
7-azanorbornadiene
cis-1,2-dichloroethylene (156-59-2)
p-Tolyl 2-(trimethylsilyl)ethynyl sulfone (34452-56-7)
N-methoxycarbonylpyrrole (4277-63-8)
trans-2-bromovinyl phenyl sulfone
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved