Org. Synth. 1990, 69, 31
DOI: 10.15227/orgsyn.069.0031
DIASTEROSELECTIVE FORMATION OF α-METHOXYCARBONYL LACTONES THROUGH AN INTRAMOLECULAR DIELS–ALDER REACTION: (4RS,4aRS,6RS,8aRS)-, (4S,4aS,6S,8aS)- AND (4R,4aR,6R,8aR)-4-METHOXYCARBONYL-1,1,6-TRIMETHYL-1,4,4A,5,6,7,8,8a-OCTAHYDRO-2,3-BENZOPYRONE [rac-5, (+)-5, AND (−)-5]
Submitted by L. F. Tietze, G. v. Kiedrowski, K.-G. Fahlbusch, and E. Voss
1.
Checked by Charles F. Marth and Edwin Vedejs.
1. Procedure
A.
Diels–Alder-adduct rac-3.
2 A
250-mL, round-bottomed flask equipped with a
pressure-equalizing addition funnel with a
calcium-sulfate-filled drying tube, a
nitrogen inlet, and a
magnetic stirring bar is charged with
2,2-diamethyl-1,3-dioxane-4,6-dione 2 (Meldrum's acid) ((Note 1), 10.0 g, 69.4 mmol), a catalytic amount of
ethylenediammonium diacetate (EDDA) ((Note 2), 500 mg, 2.77 mmol) and dry
methanol (150 mL).
(R,S)-Citronellal (rac-1, Sigma; dried over MgSO4 and distilled) ((Note 3), 9.74 g = 11.4 mL, 63.1 mmol) is added under
nitrogen (Note 4) over 15 min through the dropping funnel to the well-stirred mixture while the temperature is kept at 15–20°C by cooling the flask with a
water bath. The solution is stirred for an additional 45 min at room temperature, the solvent is removed on a
rotary evaporator (25°C), and the remaining yellow oil is dissolved in
diethyl ether (300 mL). The organic layer is washed with water
(50 mL), saturated sodium bicarbonate (2 × 50 mL) and
brine (50 mL), and dried over anhydrous
sodium sulfate. Filtration and removal of the solvent gives an 8 : 1 mixture (16.5 g) of the Diels–Alder adduct
rac-
3 and the ene-product
rac-
4 as a yellow oil
(Note 5).
B. Lactone 5. The crude mixture of rac-3 and rac-4 is dissolved in 300 mL of dry methanol (distilled from sodium) containing 10 drops of concentrated hydrochloric acid and heated under reflux for about 8 hr until the reactants can no longer be detected by thin-layer chromatography (Note 6). The solvent is removed on a rotary evaporator at 25°C and the remaining residue, which consists of an 8 : 1 mixture of lactone rac-5 and dimethyl ester rac-6, is dissolved in dry dichloromethane (50 mL). The solution is acidified with trifluoroacetic acid (10 mL) and stirred at room temperature for about 48 hr, until the thin-layer chromatogram does not show any dimethyl ester rac-6 (Note 6). The organic layer is washed with water (50 mL), saturated sodium bicarbonate solution (2 × 50 mL), water (50 mL), and brine (50 mL), dried over sodium sulfate, filtered, and concentrated on a rotary evaporator. Distillation of the remaining thick, yellow oil under reduced pressure in a short-path distillation apparatus with an air-cooled condenser gives 12.6 g (79%) of rac-5, bp 133–135°C at 0.001 mm. The colorless oil is dissolved in tert-butyl methyl ether (10 mL) and hexane (80 mL) and the solvent is allowed to evaporate over 2 days to about 15% of the original volume. Lactone rac-5 (8.11 g, 53%) slowly crystallizes (mp 69–71°C) (Note 7) and (Note 8). If the above procedure is repeated with the mother liquor, a variable additional amount of rac-5 (Note 8) is obtained.
With (S)-citronellal the (4S,4aS,6S,8aS)-lactone (+)-5 is obtained; with (R)-citronellal the (4R,4aR,6R,8aR)-lactone (−)-5 is obtained (Note 3) and (Note 7).
2. Notes
1.
Meldrum's acid is commercially available from Merck-Schuchardt, Fluka, or Aldrich Chemical Company, Inc., or it can be prepared by the reaction of
malonic acid with
acetone.
32.
Ethylenediammonium diacetate (EDDA) is prepared as follows.
4 A
250-mL, round-bottomed flask with a stirring bar and a pressure-equalizing addition funnel with a calcium-sulfate-filled drying tube is charged with dry
ethylenediamine (12.0 g. 0.20 mol) and dry
ether (100 mL).
Acetic acid (24.0 g, 0.40 mol) in dry
ether (20 mL) is added through the dropping funnel to the stirred solution. The reaction mixture is left at 4°C for 14 hr and the crystals are collected by filtration and washed with ether. Recrystallization from
methanol provides
19.8 g (
83%) of pure EDDA, mp
114°C, as white needles; IR (KBr) cm
−1: 3500–2000 (NH), 2180 (MH
3+), 1650 (C=O), 1600–1400 (CO
2−);
1H NMR (CDCl
3) δ: 1.90 (s, 6 H, CH
3), 3.20 (s, 4 H, CH
2), 5.75 (s, 6 H, NH
3+).
EDDA is the best catalyst for the condensation. Piperidine acetate gives side products.
3.
(R,S)-Citronellal can be purchased from BASF; and
(R)-citronellal, from Dragoco, Fluka, or Takasgo Perfumery Co., Ltd., Japan.
(R)-Citronellal can also be synthesized from
pulegone with ee >99%.
5 (S)-Citronellal may be obtained by oxidation of
(S)-citronellol,
6 which is accessible by different routes with ee 95%.
7 The optical purity of
citronellal can be determined by GLC after conversion to the acetal of
(−)-(2R,4R)-pentanediol.
8 For the reactions described,
(R,S)-citronellal from BASF,
(R)-citronellal from Dragoco, and
(S)-citronellol from Fluka were used.
(R,S)-Citronellal and
(S)-citronellal were distilled under
nitrogen before use (bp 83–85°C/11 mm),
(S)-citronellal:
[α]D20 −11.5° (
chloroform,
c 0.1);
(R)-citronellal (
[α]D20 + 13 ± 1°) and (
S)-citronellal (
[α]D20 −4.9 ± 0.2°) were used as purchased.
4.
The reaction can also be performed without using inert gas, but the yields may be lower.
5.
The pure Diels–Alder adduct
3 can be obtained by crystallization of the crude reaction product from
ether/hexane: white needles, mp
104–106°C; IR (KBr) cm
−1; 2950, 2930, 2860 (C-H), 1715 (C=O), 1615 (C=C), 1400, 1265;
1H NMR (CDCl
3) δ: 0.40 (m, 1 H, 4 β-H), 0.7–2.5 (m, 7 H, CH + CH
2), 0.90 (d, 3 H,
J = 7, CH
3), 1.23, 1.43, 1.70, 1.73 (s, 3 H, CH
3), 2.75 (dt, 1 H,
J1 = 12,
J2 = 2, 4-H). When the pure Diels–Alder adduct
3 is heated in dry
methanol under reflux for 3 hr,
5 (mp
68–70°C) is obtained in
92% yield from
3.
6.
Macherey-Nagel Polygram SIL G/UV
254-plates were used with 2 : 5 v/v
ether/
hexane as eluant. The Diels–Alder product
3 (
Rf = 0.29), is visible under short-wavelength ultraviolet light, whereas the detection of
4 (
Rf = 0.33),
rac-
5 (
Rf = 0.22) and
6 (
Rf = 0.47) is effected by development in an
iodine chamber.
7.
The physical properties of
rac-
5, (+)-
5, and (−)-
5 are as follows: (+)-
5,
[α]D20 +44.1° (
chloroform,
c 1.004); (−)-
5,
[α]D20 −44.0°, (
chloroform,
c 0.995); IR (KBr) cm
−1: 2980, 2950, 2930, 2870, (CH), 1745, 1725 (C=O), 1450, 1320;
1H NMR (200 MHz, CDCl
3) δ: 0.74 (ddd, 1 H,
J = 12, 12, 12, 5β-H), 0.86–1.7 (m, 5 H, 6, 7β, 8a, 8α, 8β-H), 0.95 (d, 3 H,
J = 6.5, 6-CH
3), 1.36 (s, 3 H, 1α-CH
3), 1.7–1.9 (m, 2 H, 5α, 7α-H), 1.42 (s, 3 H, 1β-CH
3), 2.16 (dddd, 1 H,
J = 3.5, 12, 12, 12, 4a-H), 3.09 (d, 1 H,
J = 12, 4-H), 3.81 (s, 3 H, OCH
3); >
13C NMR (50.3 MHz, CDCl
3) δ: 22.0 (1α-CH
3), 23.3 (6-CH
3), 27.2 (C-7), 28.2 (1β-CH
3), 31.6, (C-6), 34.2 (C-8), 36.0 (C-8a), 40.5 (C-5), 45.9 (C-4a), 52.6 (OCH
3), 55.1 (C-4), 86.6 (C-1), 167.1 (C=O), 169.6 (C-3); MS (70 eV):
m/e = 254 (1%, M
+), 239 (6%, M–CH
3), 223 (2%, M–OCH
3), 196 (50%, M–C
3H
6O), 168 (15%, 196-CO), 109 (22%, 168-CO
2CH
3), 101 (100%), 59 (55%, CO
2CH
3).
8.
Crystallization of the crude material without distillation from
tert-butyl methyl ether/hexane affords
56% of
rac-
5, mp
68–70°C, as pale-yellow crystals. The submitters obtained a second crop of
1.5 g from crystallization of distilled material; mp
68–78°C, starting from
citronellal purchased from BASF. The checkers found that
citronellal from Sigma required distillation and gave an impure second crop of
5 only with difficulty.
3. Discussion
Lactone
5 can be obtained in both enantiomeric forms or as racemate according to the described procedure. The reaction sequence includes the in situ formation of an alkylidene-1,3-dicarbonyl system
7, which can act as a heterodiene in an intramolecular hetero-Diels–Alder addition. A small amount of the ene product
4 with diastereomeric excess (de) > 98% is formed at room temperature as well. The remarkable selectivity in formation of diastereomer
3 is explained by an energetically more favorable
exo transition state
8 with a pseudochair arrangement having the methyl group quasiequatorial. Polycyclic
cis-fused compounds can also be synthesized by the procedure above,
9 and a related sequence to the cannabinoid skeleton has been described using appropriate 1,3-dicarbonyl reactants.
10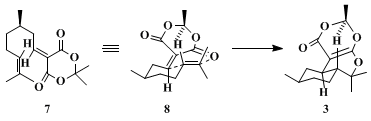
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
(4RS,4aRS,6RS,8aRS)-, (4S,4aS,6S,8aS)- AND (4R,4aR,6R,8aR)-4-METHOXYCARBONYL-1,1,6-TRIMETHYL-1,4,4A,5,6,7,8,8a-OCTAHYDRO-2,3-BENZOPYRONE [rac-5, (+)-5, AND (−)-5]
2,2-diamethyl-1,3-dioxane-4,6-dione
(−)-(2R,4R)-pentanediol
hydrochloric acid (7647-01-0)
acetic acid (64-19-7)
methanol (67-56-1)
ether,
diethyl ether (60-29-7)
chloroform (67-66-3)
sodium bicarbonate (144-55-8)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
acetone (67-64-1)
sodium (13966-32-0)
dichloromethane (75-09-2)
Malonic acid (141-82-2)
MgSO4 (7487-88-9)
ethylenediamine (107-15-3)
hexane (110-54-3)
piperidine acetate
trifluoroacetic acid (76-05-1)
citronellal (106-23-0)
MELDRUM'S ACID (2033-24-1)
pulegone (89-82-7)
ethylenediammonium diacetate
(S)-Citronellol (7540-51-4)
(R)-Citronellal,
(R,S)-citronellal (2385-77-5)
tert-butyl methyl ether (1634-04-4)
(S)-citronellal
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved