Org. Synth. 1988, 66, 87
DOI: 10.15227/orgsyn.066.0087
CYCLOPENTANONES FROM CARBOXYLIC ACIDS VIA INTRAMOLECULAR ACYLATION OF ALKYLSILANES: 2-METHYL-2-VINYLCYCLOPENTANONE
[Cyclopentanone, 2-ethenyl-2-methyl-]
Submitted by Isao Kuwajima and Hirokazu Urabe
1.
Checked by Robert J. Ross and Leo A. Paquette.
1. Procedure
Caution! The following reactions should be performed in an efficient hood.
A. 1-Iodo-3-trimethylsilylpropane. In a dry, 100-mL, two-necked, round-bottomed flask equipped with a magnetic stirring bar, a reflux condenser, and a rubber septum is placed 15.0 g (0.10 mol) of sodium iodide. A nitrogen inlet tube is connected to the top of the reflux condenser and all the apparatus is kept under nitrogen. To this vessel are added 50 mL of acetone and 11.5 mL (10 g, 0.066 mol) of 1-chloro-3-trimethylsilylpropane (Note 1) with a hypodermic syringe through the septum; the resulting white suspension is stirred under reflux for 24 hr. The condenser is replaced with a Claisen head, and the bulk of the solvent is removed under ordinary pressure (Note 2) to give a white slurry. To this is added 60 mL of hexane and the inorganic salts are filtered off by suction. The filter cake is washed with five 10-mL portions of hexane. The hexane is distilled off from the combined organic portions at atmospheric pressure. The residual oil is transferred to a 50-mL, round-bottomed flask fitted with a stirring bar and a Claisen head, and is distilled under reduced pressure to afford 1-iodo-3-trimethylsilylpropane 1 (11.5–13.1 g, 72–81%), bp 84–86°C (25 mm), as a clear liquid (Note 3) and (Note 4).
B. 2-Methyl-2-vinylcyclopentanone. A 300-mL, two-necked, round-bottomed flask fitted with a magnetic stirring bar, a nitrogen inlet tube, and a rubber septum is kept under dry nitrogen. To this flask are introduced 8.3 mL (5.99 g, 0.0591 mol) of diisopropylamine and 120 mL of tetrahydrofuran (Note 5) with a syringe through the septum. The flask is cooled in a dry ice–hexane bath. To the solution is slowly added 38.7 mL of butyllithium (1.53 M in hexane, 0.0592 mol) with a syringe and the mixture is kept at 0°C (in an ice bath) for 10 min. The resulting solution of lithium diisopropylamide is again cooled in a dry ice–hexane bath (−78°C), and 11.2 mL (11.57 g, 0.0645 mol) of N,N'-dimethylpropyleneurea (DMPU) (Note 6) is added. After stirring for 30 min, 6.47 mL (6.15 g, 0.0539 mol) of methyl tiglate is injected drop by drop at −78°C. After the solution is stirred for an additional 20 min, 13.0 g (0.0537 mol) of 1-iodo-3-trimethylsilylpropane 1 is added with a syringe and the dry ice–hexane bath is replaced with an ice bath. The solution is stirred at about 0°C for 1 hr and then poured onto 150 mL of ice-cooled 3 N hydrochloric acid covered with 150 mL of hexane. The organic layer is separated and the aqueous layer is extracted with two 80-mL portions of hexane. The combined organic layers are washed successively with 50 mL of 3 N hydrochloric acid and 50 mL of saturated sodium bicarbonate solution and dried over anhydrous magnesium sulfate. The solvent is removed on a rotary evaporator to afford the crude ester 2 (ca. 13 g) (Note 7), which is sufficiently pure for the next operation.
In a two-necked, round-bottomed flask equipped with a magnetic stirring bar, a rubber septum, and a reflux condenser, the top of which is fitted with a nitrogen inlet tube, is placed 7.3 g (ca. 0.13 mol) of 85% pure potassium hydroxide. To the flask are added 4 mL of water and an ethanol solution (60 mL) of the crude ester 2 (ca. 13 g) with a syringe; the mixture is refluxed for 1 hr. The reflux condenser is replaced with a Claisen head and the bulk of the solvent is distilled off over 30 min (Note 8). The residue is cooled in an ice bath and 80 mL of 6 N hydrochloric acid is cautiously introduced. The mixture is extracted with 150 mL of hexane and the organic layer is separated. To the aqueous layer is added 40 mL of concentrated hydrochloric acid and the solution is again extracted with two 100-mL portions of hexane. The combined organic extracts are dried over anhydrous magnesium sulfate and concentrated under reduced pressure to afford the crude acid 3 (10.6–12.0 g) as a dark-colored oil (Note 9).
A 200-mL, two-necked, round-bottomed flask fitted with a magnetic stirring bar, a reflux condenser, and a rubber septum is flushed with nitrogen. To this flask is introduced a dry benzene solution (50 mL) of crude acid 3 through the septum. Then 8.9 mL (12.9 g, 0.101 mol) of oxalyl chloride is slowly added with stirring at room temperature. After the evolution of gas ceases, the solution is further heated in an oil bath maintained at 70°C for 30 min. The solvent, together with excess oxalyl chloride, is removed at room temperature first on a rotary evaporator and finally with a vacuum pump to leave the crude acid chloride of 3 as a dark-colored oil (Note 10).
In a 300-mL, three-necked, round-bottomed flask fitted with an nitrogen inlet tube, a dropping funnel, and a rubber septum are placed 7.45 g (0.0559 mol) of powdered aluminum chloride and a magnetic stirring bar. After 100 mL of dichloromethane (Note 11) is introduced, the crude acid chloride, dissolved in 50 mL of dichloromethane, is added via the dropping funnel to the stirred suspension of aluminum chloride at 0°C over 5 min, whereupon most of the aluminum chloride dissolves. After further stirring at 0°C for 15 min and at room temperature for 15 min, the flask is recooled in an ice bath and 100 mL of 3 N hydrochloric acid is cautiously added through the dropping funnel. The organic phase is separated and the aqueous layer is extracted 3 times with 50-mL portions of dichloromethane. The combined organic layers are successively washed with 30 mL of 3 N hydrochloric acid and 50 mL of saturated sodium bicarbonate solution, and dried over anhydrous magnesium sulfate. The solvent is removed under ordinary pressure and the residue is distilled under reduced pressure to give 2-methyl-2-vinylcyclopentanone as a clear liquid (3.74–5.6 g, 56–84% yield based on the methyl tiglate), bp 104–124°C (110 mm) (Note 12), ca. 95% pure by GLC (Note 13).
2. Notes
1.
1-Chloro-3-trimethylsilylpropane, obtained from Petrarch Systems, Inc., was used as received.
2.
About 40 mL of distillate is collected.
3.
1-Iodo-3-trimethylsilylpropane has the following spectral properties:
1H NMR (CCl
4, 3% benzene (δ 7.24) as an internal standard) δ: −0.03 (s, 9 H, (CH
3)
3Si), 0.34–0.74 (m, 2 H, CH
2Si), 1.47–2.04 (m, 2 H, CH
2), 3.07 (t, 2 H,
J = 7, CH
2I); IR (neat) cm
−1: 2950, 1250, 860, 830.
4.
1-Iodo-3-trimethylsilylpropane can also be prepared by other methods;
2.
5.
Tetrahydrofuran was used after distillation from
sodium benzophenone ketyl under
nitrogen.
6.
The submitters used
hexamethylphosphoric triamide (HMPA), but it was replaced for safety reasons by
N,N'-dimethylpropyleneurea (DMPU),3 obtained from Aldrich Chemical Company, Inc. or Fluka; no change in procedure was required and the yield was unaffected.
7.
Alkylation of
methyl tiglate was carried out according to a reported procedure.
48.
About 50 mL of distillate was collected.
9.
Crude
3 exhibited the following spectral properties, which are virtually identical to those of an analytically pure sample:
1H NMR (CCl
4, 3% benzene (δ 7.24) as an internal standard) δ: 0.16 (s, 9 H, (CH
3)
3Si), 0.44–0.77 (m, 2 H, CH
2Si), 1.1–2.0 (m, 4 H, CH
2CH
2), 1.41 (s, 3 H, CH
3), 4.91–5.31 (m, 2 H, C=CH
2), 6.04 (d of d, 1 H,
J = 10 and 18, CH=CH
2), 11.34 (s, 1 H, CO
2H); IR (neat) cm
−1: 2950, 1700, 1400, 1250, 1180, 920, 840, 740, 680.
10.
Conversion of the carboxylic acid to the acid chloride was based on a reported method.
511.
Dichloromethane was distilled from
phosphorus pentoxide.
12.
2-Methyl-2-vinylcyclopentanone showed the following spectral properties:
1H NMR (CCl
4) δ: 0.73 (s, 3 H, CH
3), 1.6–2.3 (m, 6 H, (CH
2)
3), 4.67–5.07 (m, 2 H, C=CH
2), 5.62 (d of d, 1 H,
J = 8 and 18, CH=CH
2); IR (neat) cm
−1: 3070, 2950, 1730, 1640, 1450, 1400, 1150, 1060, 1040, 1000, 920.
13.
Vapor-phase chromatography was performed on an OV 101, fused silica, 20-m capillary column.
3. Discussion
Cyclopentanones are widely found in natural products and are also useful intermediates in organic synthesis. Thus a facile construction of cyclopentanones from easily available acyclic precursors is particularly desirable. This method of preparation is based on an intramolecular acylation of 5-trimethylsilylalkanoyl chlorides previously reported by us.
2 The starting materials are generally prepared by alkylation of carboxylic acids with 3-trimethylsilylalkyl halides followed by their conversion to the corresponding acid chlorides. The cyclization of the acid chlorides proceeds cleanly with
aluminum chloride. An acyl cation generated from the acid chloride and
aluminum chloride is trapped with the alkyl–silicon bond in the same molecule to yield a cyclopentanone selectively:
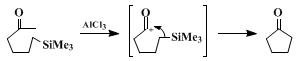
Other results are collected in Table I,
2 which shows that cyclopentanones having a variety of substituents can be prepared according to this procedure with substantial advantage over other methods, such as alkylation of 2-alkoxycarbonylcyclopentanone.
TABLE I
CYCLOPENTANONE SYNTHESIS BY INTRAMOLECULAR ACYLATION OF 5-TRIMETHYLSILYLALKANOYL CHLORIDESa
|
|
|
|
Acid (1)
|
Product (3)
|
Yield(%)b
|
|
|
|
|
92c
|
|
|
|
83
|
|
|
|
84
|
|
|
|
85
|
|
|
|
60d
|
|
|
|
67
|
|
|
|
70
|
|
|
|
76e
|
|
aReactions are carried out on 0.2–0.3-mmol scale with the reactant ratio 1/(COCl)2/AlCl3 = 1 : 2 : 1.
|
bOverall yield from 1. Products are isolated by chromatography.
|
cReaction on 3.5-mmol scale; the product was isolated by Kugelrohr distillation.
|
dReactant ratio: 1/(COCl)2/AlCl3 = 1:1.5:0.75.
|
eReactant ratio: 1/(COCl)2/AlCl3 = 1:2:2.
|
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
sodium benzophenone ketyl
ethanol (64-17-5)
hydrochloric acid (7647-01-0)
Benzene (71-43-2)
sodium bicarbonate (144-55-8)
nitrogen (7727-37-9)
acetone (67-64-1)
aluminum chloride (3495-54-3)
potassium hydroxide (1310-58-3)
sodium iodide (7681-82-5)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
oxalyl chloride (79-37-8)
hexane (110-54-3)
hexamethylphosphoric triamide (680-31-9)
lithium diisopropylamide (4111-54-0)
diisopropylamine (108-18-9)
N,N'-dimethylpropyleneurea (7226-23-5)
phosphorus pentoxide (1314-56-3)
2-Methyl-2-vinylcyclopentanone,
Cyclopentanone, 2-ethenyl-2-methyl- (88729-76-4)
1-chloro-3-trimethylsilylpropane (2344-83-4)
1-Iodo-3-trimethylsilylpropane (18135-48-3)
methyl tiglate (6622-76-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved