Org. Synth. 1992, 70, 111
DOI: 10.15227/orgsyn.070.0111
(E)-1-BENZYL-3-(1-IODOETHYLIDENE)PIPERIDINE: NUCLEOPHILE-PROMOTED ALKYNE-IMINIUM ION CYCLIZATIONS
[Piperidine, 3-(iodoethylidene)-1-(phenylmethyl)-(E)-]
Submitted by H. Arnold, L. E. Overman, M. J. Sharp, and M. C. Witschel
1.
Checked by Antje Grützmann, Thomas Hache, and Ekkehard Winterfeldt.
1. Procedure
A. 4-Hexyn-1-yl methanesulfonate (1). An oven-dried, 500-mL, one-necked, round-bottomed flask equipped with a magnetic stirring bar is flushed with argon and 260 mL of dichloromethane is added (Note 1). The flask is sealed with a rubber septum inlet and cooled to ca. −10°C in an ice-salt bath. To this flask are added via syringe 11 mL (80 mmol) of triethylamine, 5.0 g (51 mmol) of 4-hexyn-1-ol (Note 2), and 4.3 mL (56 mmol) of methanesulfonyl chloride (Note 3). The resulting solution is stirred for an additional 30 min and then quenched by adding 30 mL of ice-water. The organic layer is separated and washed successively with 1 M hydrochloric acid solution (30 mL), saturated aqueous sodium bicarbonate solution (30 mL), and brine (30 mL). The organic layer is dried over magnesium sulfate, filtered, and concentrated with a rotary evaporator to give 8.3–9.0 g (93–100%) of crude 4-hexyn-1-yl methanesulfonate (1) which was used directly in the next step (Note 4).
B. N-Benzyl-4-hexyn-1-amine (2). An oven-dried, 100-mL, one-necked, round-bottomed flask containing a magnetic stirring bar and a rubber septum inlet is flushed with argon and charged with 200 mg of sodium iodide. The crude mesylate 1, 40 mL of dimethyl sulfoxide (Note 5) and 10.9 g (102 mmol) of benzylamine are added via syringe. The resulting solution is heated in an oil bath at 47–53°C for 5 hr (Note 6) and then allowed to cool to room temperature. The reaction solution is poured into a separatory funnel containing 200 mL of aqueous 1% sodium hydroxide solution and the resulting mixture is extracted with ether (3 × 100 mL). The combined ether extracts are washed with brine (50 mL), dried over magnesium sulfate, filtered, and concentrated with a rotary evaporator. The residue (ca. 9 g) is purified by flash chromatography (ca. 300 g of silica gel using 1:1 hexane-ether containing 5% triethylamine as the eluent) (Note 7) to give 7.2–7.5 g (75–79% overall) of 2 as a colorless liquid (Note 8).
C. (E)-1-Benzyl-3-(1-iodoethylidene)piperidine (3). A 250-mL, one-necked, round-bottomed flask containing a magnetic stirring bar and a reflux condenser topped with a rubber septum inlet is flushed with argon and charged with 4.0 g (21 mmol) of alkynylamine 2, 11 g (73 mmol) of sodium iodide (Note 9), 35 mL of 37% w/w formaldehyde solution, 5.4 g (22 mmol) of camphorsulfonic acid monohydrate (Note 10) and 80 mL of water. The resulting mixture is heated at reflux under an argon atmosphere for 15 min (Note 11) and then allowed to cool to room temperature. This solution is made basic by adding 5 M aqueous potassium hydroxide solution and then poured into a separatory funnel where it is extracted with dichloromethane (3 × 50 mL) (Note 12). The combined organic layers are dried over sodium sulfate, filtered, and concentrated with a rotary evaporator. The resulting residue is purified by flash chromatography (ca. 150 g of silica gel, 1:1 hexane-ethyl ether containing 5% triethylamine as eluent) to give 5.4–6.2 g (79–90%) of 3 as a colorless oil (Note 13) and (Note 14).
2. Notes
1.
Dichloromethane is distilled from
calcium hydride (CaH
2) and added directly from the still to the reaction flask.
2.
This alcohol is readily prepared in standard fashion from the tetrahydropyranyl ether of
4-pentyn-1-ol and
iodomethane: A
hexane solution of
butyllithium (46 mL of a 2.2 M solution, 100 mmol) is added dropwise under an
argon atmosphere to a dry ice-cooled solution of
tetrahydro-2-(4-pentynyloxy)-2H-pyran (14.4 g, 84 mmol, prepared from commercially available
4-pentyn-1-ol2) in
100 mL of dry tetrahydrofuran. After 10 min,
7.0 mL (110 mmol) of iodomethane is added dropwise to the dry ice-cooled, stirring solution of the alkynyllithium intermediate. The reaction mixture is maintained at dry ice temperature for 1 hr, and, after warming to room temperature, the
tetrahydrofuran is removed by mild rotary evaporation (or distillation at atmospheric pressure). The crude product is dissolved in
100 mL of ether and washed with
brine (50 mL), and the aqueous phase is back-extracted with
ether (25 mL). The combined organic phases are concentrated and the residue is dissolved in
200 mL of methanol.
p-Toluenesulfonic acid (4 g) is added and the resulting solution is heated at reflux for 3 hr. The solvent is removed by distillation through an
8–10 cm Vigreux column, the residue is dissolved in
100 mL of ether and this solution is extracted with
25 mL of aqueous 10% sodium carbonate solution. After the solution is dried over MgSO
4, the solvent is removed by distillation and the residue is distilled through a
50-cm concentric tube column. The fraction boiling at
92–93°C (10 mm Hg) is collected to give
7.8 g (
94%) of
4-hexyn-1-ol, which is >95% pure by GC analysis (
30 m, Supelco SPB-5 capillary column).
3.
Triethylamine, distilled from CaH
2, and
methanesulfonyl chloride, vacuum distilled at ca. 60°C (20 mm), are employed.
4.
The spectrum is as follows:
1H NMR (300 MHz, CDCl
3) δ: 1.78 (t, 3 H, J = 2.5), 1.91 (apparent pentaplet, 2 H, J = 6.5), 2.25–2.35 (m, 2 H), 3.03 (s, 3 H), 4.35 (t, 2 H, J = 6.1).
5.
Dimethyl sulfoxide, distilled at 20 mm from CaH
2, and
benzylamine, freshly distilled at 20 mm, are employed.
6.
The reaction is easily monitored by TLC (silica gel, 1:1
ether-
ethyl acetate): mesylate R
f = 0.9, amine R
f = 0.4.
7.
This material can also be purified by vacuum distillation; however, some decomposition results.
8.
This material is 97% pure by capillary GC analysis (30 m, J & W DB-5 fused silica column). Spectral data are as follows: IR (film) cm
−1: 3330, 1453, 1120, 736;
1H NMR (300 MHz, CDCl
3) δ: 1.69 (apparent pentaplet, 2 H, J = 7), 1.77 (t, 3 H, J = 2.5), 2.15–2.25 (m, 2 H), 2.73 (t, 2 H, J = 7.0), 3.80 (s, 2 H), 7.2–7.4 (m, 5 H); MS (isobutane CI): 188 (MH), 172, 120, 91; high resolution MS (70 eV, EI) 187.1331 (187.1261 Calcd for C
13H
17N).
9.
Fisher Certified sodium iodide is used as received.
10.
Fisher Certified A.C.S. formaldehyde solution is used as received.
Aldrich Chemical Company, Inc., camphorsulfonic acid monohydrate is recrystallized from
ethyl acetate prior to use.
11.
This conversion is easily monitored by TLC (silica gel, 1:1
hexane-
ethyl acetate containing
5% triethylamine):
2, R
f = 0.4,
3, R
f = 0.7.
12.
The free base of this
iodoamine darkens slowly when exposed to room light. The isolation procedure should be conducted rapidly or the separatory funnel and rotary evaporator bulb should be wrapped in
aluminum foil to exclude room light.
13.
This material is at least 95% pure by capillary GC analysis (30 m, J & W DB-5 fused silica column); a small unknown impurity (ca. 2%) with characteristic
1H NMR signals at δ 3.41, 4.77 and 4.92 is apparent in some chromatography fractions. Spectral data for
3 are as follows: IR (film) cm
−1: 1646, 1228, 1138, 1119, 1061, 739;
1H NMR δ: 1.55–1.8 (m, 2 H), 2.40 (t, 2 H, J = 6.3), 2.45 (s, 3 H), 2.56 (t, 2 H, J = 5.5), 3.10 (s, 2 H), 3.57 (s, 2 H), 7.2–7.4 (m, 5 H); MS (isobutane CI): 328 (MH), 202, 200, 112, 110, 92. High resolution MS (70 eV, EI): 327.0465 (327.0484 calcd for C
14H
18NI).
14.
The maleate salt is prepared in good yield and crystallizes as fine needles (ca. 1 g of salt/10 mL) from absolute
ethanol: mp
143–144°C. This salt can be stored at room temperature in room light with no noticeable decomposition. Anal. Calcd. for C
18H
22INO
4: C, 48.77; H, 5.00; N, 3.16. Found: C, 48.70; H, 5.00; N, 3.09.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Simple alkynes do not undergo intramolecular reactions with weakly electrophilic iminium ions in the absence of strong external nucleophiles.
3 For example, the formaldiminium ion derived from
4-hexynylamine,
formaldehyde, and
camphorsulfonic acid does not cyclize when maintained in
acetonitrile at 100°C for 1 hr.
3 Iminium ion-alkyne cyclizations do take place in nucleophilic solvents such as H
2O
4 or in non-nucleophilic solvents when a strong nucleophile is present.
3The present procedure illustrates the use of added iodide anion to promote the Mannich cyclization of an alkyne to afford 3-alkylidenepiperidines. As illustrated in Table I a variety of nonbasic nucleophiles with nucleophilic constants
5 η-CH
3I >5.8 are useful promoters of formaldiminium ion-alkyne cyclizations.
3 Piperidines containing both endocyclic and exocyclic allylic unsaturation can be efficiently assembled in this way from readily available alkynol precursors (see Table I).
3 To the limits of
1H NMR detection at 500 MHz all nucleophile-promoted cyclizations that form 3-alkylidenepiperidines occur with complete anti-stereoselectivity.
TABLE I
NUCLEOPHILE-PROMOTED IMINIUM ION-ALKYNE CYCLIZATIONS3
|
|
|
|
R'
|
R
|
X
|
Yield
|
|
|
OCH3
|
CH3
|
Br
|
89%
|
|
OCH3CH3
|
CH3
|
I
|
80%
|
|
OCH3
|
n-Bu
|
Br
|
63%6
|
|
OCH3
|
n-Bu
|
I
|
76%6
|
|
H
|
CH3
|
I
|
81-90%
|
|
H
|
CH3
|
N3
|
72%
|
|
H
|
CH3
|
SCN
|
82%
|
|
OCH3
|
H
|
I
|
56%
|
|
OCH3
|
CH3
|
SPh
|
<15%
|
|
|
CH3
|
Br
|
75%
|
|
|
CH3
|
I
|
90%
|
|
|
CH3
|
N3
|
40%
|
|
|
Me3Si
|
Br
|
44%
|
|
|
Me3Si
|
I
|
60%
|
|
|
H
|
I
|
87%
|
|
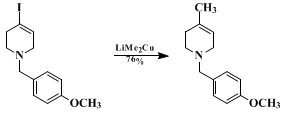
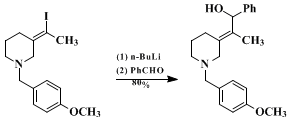
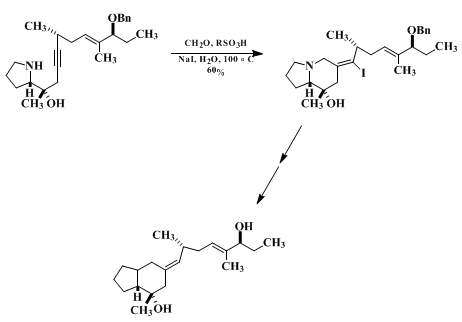
The usefulness of nucleophile-promoted iminium ion-alkyne cyclizations derives from the ready availability of alkynylamines and the subsequent transformations of the cyclization products made possible because of their vinylic functionality (e.g., equations 1 and 2). Equation 2 illustrates use of this chemistry to elaborate an exocyclic tetrasubstituted double bond with complete stereocontrol. Net "reductive" iminium ion alkyne cyclizations can be accomplished by dehalogenation of vinyl halide cyclization products. The conversion illustrated in equation 3 is a key step in an efficient, practical synthesis of the cardiotonic frog alkaloid pumiliotoxin A.
7
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
ethanol (64-17-5)
hydrochloric acid (7647-01-0)
ethyl acetate (141-78-6)
methanol (67-56-1)
ether,
ethyl ether (60-29-7)
acetonitrile (75-05-8)
sodium hydroxide (1310-73-2)
formaldehyde (50-00-0)
sodium bicarbonate (144-55-8)
sodium carbonate (497-19-8)
sodium sulfate (7757-82-6)
aluminum (7429-90-5)
potassium hydroxide (1310-58-3)
iodomethane (74-88-4)
sodium iodide (7681-82-5)
dichloromethane (75-09-2)
magnesium sulfate (7487-88-9)
benzylamine (100-46-9)
butyllithium (109-72-8)
Tetrahydrofuran (109-99-9)
hexane (110-54-3)
dimethyl sulfoxide (67-68-5)
triethylamine (121-44-8)
argon (7440-37-1)
calcium hydride (7789-78-8)
Methanesulfonyl chloride (124-63-0)
mesylate (75-75-2)
4-Pentyn-1-ol (5390-04-5)
camphorsulfonic acid (5872-08-2)
p-toluenesulfonic acid (104-15-4)
(E)-1-Benzyl-3-(1-iodoethylidene)piperidine (146980-71-4)
Piperidine, 3-(iodoethylidene)-1-(phenylmethyl)-(E)-
4-Hexyn-1-yl methanesulfonate (68275-05-8)
4-hexyn-1-ol (928-93-8)
N-Benzyl-4-hexyn-1-amine (112069-91-7)
camphorsulfonic acid monohydrate (5872-08-2)
tetrahydro-2-(4-pentynyloxy)-2H-pyran (62992-46-5)
iodoamine
4-hexynylamine
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved