Org. Synth. 1992, 70, 54
DOI: 10.15227/orgsyn.070.0054
A STABLE CHIRAL 1,4-DIHYDROPYRIDINE EQUIVALENT FOR THE ASYMMETRIC SYNTHESIS OF SUBSTITUTED PIPERIDINES: 2-CYANO-6-PHENYLOXAZOLOPIPERIDINE
[5H-Oxazolo[3,2-a]pyridine-5-carbonitrile, hexahydro-3-phenyl-, [3R-(3α,5β,8aβ]]
Submitted by Martine Bonin, David S. Grierson, Jacques Royer, and Henri-Philippe Husson
1.
Checked by Gilbert Rishton and Larry E. Overman.
1. Procedure
CAUTION! Aqueous potassium cyanide is used in this procedure. All operations should be conducted in a well-ventilated hood and rubber gloves should be worn.
2-Cyano-6-phenyloxazolopiperidine. A 2-L, round-bottomed flask is charged with 10 g (0.073 mol) of (−)-phenylglycinol (Note 1) and 40 g of citric acid in 1 L of distilled water. The mixture is stirred magnetically until complete dissolution is achieved and is then cooled to 0–5°C (ice-water bath). The flask is equipped with a dropping funnel and 45 mL of an aqueous 24% glutaraldehyde solution (0.11 mol) is added dropwise over 20 min and the resultant cloudy solution is stirred for an additional 30 min at 0°C. The cooling bath is removed and a solution of 7.15 g (0.11 mol) of potassium cyanide in 20 mL of water is carefully added in one portion followed by 200 mL of methylene chloride. The resulting two-phase reaction system is stirred for 3 hr at room temperature, then the aqueous phase is neutralized (Note 2) by addition of sodium bicarbonate and the two layers are separated. The water layer is extracted with three 200-mL portions of methylene chloride (Note 3) and the combined methylene chloride layers are dried over sodium sulfate and concentrated on a rotary evaporator to a volume of 500 mL. Zinc bromide (2 g) is added in small portions over 5 min to this solution and vigorous stirring under nitrogen is continued for 3 hr (Note 4). The reaction mixture is concentrated to a volume of approximately 150 mL (Note 5) and the resultant mixture is applied to a 10-cm diameter flash chromatography column prepared using hexane-ether (2:1) as the eluant. The desired product is eluted first (Note 6), (Note 7). By recrystallization from hexane, the product [10.8–11.6 g (65–70%)] is obtained analytically pure; mp 79–81°C, [α]D23 −280° (CHCl3, c 1.0) (Note 8).
2. Notes
1.
The checkers used
(R)-(−)-2-phenylglycinol [98%, [α]D24 −31.7° (1 N HCl, c 0.76)] purchased from Aldrich Chemical Company, Inc. The submitters employed material [mp
78°C,
[α]D20 −26.5° (MeOH,
c 0.7)] prepared by
lithium aluminum hydride reduction of
(−)-phenylglycine and report that the yield of
2-cyano-6-phenyloxazolopiperidine prepared from this material is
75–83%.
2.
Extraction at pH greater than 9 led to the formation of by-products
(2,6-dicyanopiperidines)2 and consequently to a lower yield of the desired product.
3.
The aqueous layer containing residual
potassium cyanide is destroyed by addition of
potassium permanganate.
4.
The reaction must be carried out in a well-ventilated hood as
hydrogen cyanide may be evolved.
5.
Complete evaporation of the solvent gives a viscous oil which is only slightly soluble in either the elution solvent or
methylene chloride. Significant loss of material thus occurs during the purification process.
6.
R
f = 0.6 (SiO
2,
hexane-
ether : 2–1) for the product.
7.
Further elution permits the isolation of a mixture of two other isomers (0.3 g, 3.6%).
8.
The product obtained by this procedure shows the following spectral data: IR (CHCl
3) cm
−1: 2100;
1H NMR (400 MHz, CDCl
3) δ: 1.5–2.0 (m, 5 H), 2.13 (dd, 1 H, J = 11.5, J' = 1.5), 3.74 (t, 1 H, J = 7.8), 3.85 (bd, 1 H, J = 7.1), 3.90 (t, 1 H, J = 8.0), 4.12 (dd, 1 H, J = 9.7, J' = 2.8), 4.25 (t, 1 H, J = 7.9), 7.4 (m, 5 H);
13C NMR (15 MHz, CDCl
3) δ: 19.3, 28.0, 30.0, 47.4, 63.9, 73.0, 89.9, 116.0, 128.2, 128.6, 129.0, 137.4.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The preparation described here is an improvement of the previous procedure.
3 The double condensation of
glutaraldehyde with the amino group of
(R)-(−)-phenylglycinol (related to the Robinson-Schopf condensation) probably leads to the expected product via the formation of an intermediate of type A
4 and/or B.
Trapping intermediate B with cyanide ion would lead to the final product. Formation of the four possible product isomers has been observed after short reaction periods, and equilibration of this mixture to the described product 2 has been demonstrated. In the original procedure compound 2 was obtained in 50–60% yield after prolonged (72 hr) reaction in water. In the present procedure equilibration of all intermediates and/or product isomers to the observed, thermodynamically more stable form of compound 2 is accelerated and the yield improved considerably through the use of zinc bromide as a catalyst in an organic medium.
2-Cyano-6-phenyloxazolopiperidine,
2, is a stable, chiral,
1,4-dihydropyridine equivalent, useful for the asymmetric synthesis of piperidines. Synthon
2 has been used for the asymmetric synthesis of several alkaloids or structural analogs in this laboratory and by others.
5,6 A sequence of reactions involving alkylation at the α-aminonitrile center (LDA, RX), elimination of the cyano group with concomitant opening of the oxazolidine ring (NaBH
4), and finally debenzylation (H
2, Pd/C) permits the preparation of α-alkylated piperidine
3 in 90 % overall yield (ee ≥95 %).
7 From the same synthon
2, enantiomeric
4 can also be prepared.
7 A regio- and chemoselective elimination of the cyano group is also possible giving an oxazolopiperidine intermediate which can further be used to prepare the cis- and trans-2,6-disubstituted piperidines
57 and
6.
8
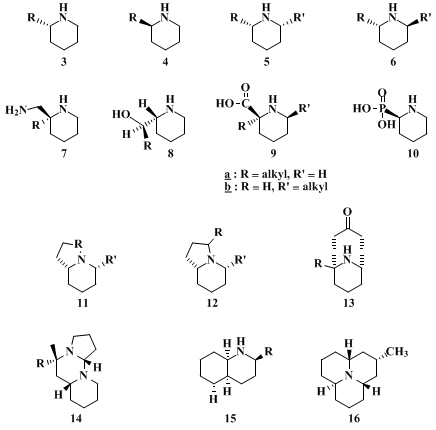
The possibility to create an R or S chiral center by substituting the CN group in 2 led us to call this methodology the CN(R,S) method, in recognition of the Centre National de la Recherche Scientifique (CNRS) which supported this work.
This methodology was extented to enantiospecific synthesis of generally less accessible or more complex piperidine derivatives :
a) 2-(1-aminomethyl)piperidines
7.
9b) Amino-alcohols
810 and other β-amino-alcohols reported in the literature.
5,6c)
Pipecolic acid and 2- or 6-alkylated derivatives
9.
11d) (R)-Enantiomer of
piperidin-2-yl phosphonic acid 10.
12e) Alkaloids
1113,
123,
1314,
1415,
1516, and the symmetric molecule
16.
17
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
2-Cyano-6-phenyloxazolopiperidine
5H-Oxazolo[3,2-a]pyridine-5-carbonitrile, hexahydro-3-phenyl-, [3R-(3α,5β,8aβ]
(−)-phenylglycinol
(−)-phenylglycine
(R)-(−)-phenylglycinol
HCl (7647-01-0)
ether (60-29-7)
citric acid (77-92-9)
sodium bicarbonate (144-55-8)
potassium permanganate (7722-64-7)
hydrogen cyanide (74-90-8)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
potassium cyanide (151-50-8)
methylene chloride (75-09-2)
lithium aluminum hydride (16853-85-3)
zinc bromide (7699-45-8)
hexane (110-54-3)
glutaraldehyde (111-30-8)
1,4-DIHYDROPYRIDINE
Pipecolic acid (535-75-1)
piperidin-2-yl phosphonic acid
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved