Org. Synth. 1997, 74, 241
DOI: 10.15227/orgsyn.074.0241
5,7-DIMETHOXY-3-METHYLINDAZOLE FROM 3,5-DIMETHOXYACETOPHENONE
[1H-Indazole, 5,7-dimethoxy-3-methyl-]
Submitted by Nicolas Boudreault and Yves Leblanc
1.
Checked by John A. McCauley and Amos B. Smith, III..
1. Procedure
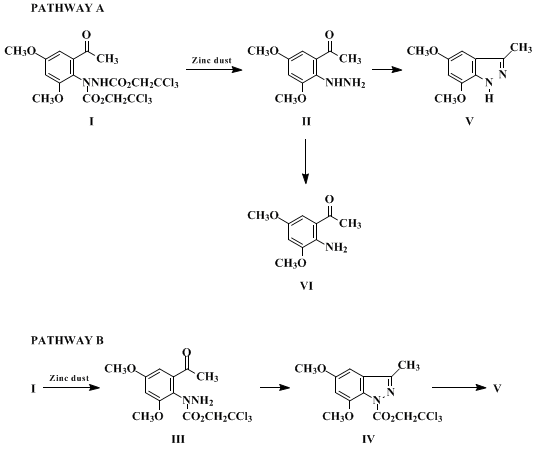
A. 1-(2-Acetyl-4,6-dimethoxyphenyl)-1,2-hydrazinedicarboxylic acid bis(2,2,2-trichloroethyl) ester. A 2-L, round-bottomed flask, equipped with a magnetic stirrer, thermometer, and an ice bath is charged with 3,5-dimethoxyacetophenone (20.00 g, 111.0 mmol) (Note 1) and dichloromethane (CH2Cl2, 555 mL) (Note 1). At 0–5°C, under nitrogen, trifluoromethanesulfonic acid (980 μL, 11.1 mmol) (Note 1) is added in one portion. To the resulting yellow solution, bis(2,2,2-trichloroethyl) azodicarboxylate (BTCEAD) (50.70 g, 133.3 mmol) (Note 2) is gradually introduced over 15 min in order to maintain an internal temperature between 0–5°C.
The orange-yellow solution is stirred for 5.5 hr at room temperature until complete reaction has taken place. The reaction mixture is poured into saturated aqueous sodium bicarbonate (NaHCO3) solution (100 mL) at 0°C. The organic phase is separated and the aqueous phase is reextracted with CH2Cl2 (30 mL). The combined organic layers are washed with water (H2O) (2 × 50 mL), dried over anhydrous sodium sulfate, filtered, concentrated and placed on a vacuum pump to afford a pale yellow foam. Ethyl acetate (85 mL) is added and the crude mixture is heated under reflux until complete dissolution occurs. At reflux hexane (85 mL) is added and the solution is allowed to stand at room temperature. After a period of 15 hr the flask containing a mass of solid is cooled in an ice bath for 1.5 hr. The solid is collected by filtration and washed with a cold ethyl acetate-hexane mixture (1:4) (50 mL). The hydrazide is dried under high vacuum at 45°C for 3 days (Note 3) to provide 52.0 g (84%) of a white solid sufficiently pure for the next step (Note 4). From the mother liquor, an additional 3.8 g (6.0%) of material is recovered by flash chromatography (20% ethyl acetate in hexane) followed by recrystallization from ethyl acetate-hexane (1:1 mixture, 20 mL).
B. 5,7-Dimethoxy-3-methylindazole. A 2-L, round-bottomed flask, equipped with a magnetic stirrer and a thermometer is charged with the hydrazide (54.0 g, 96.3 mmol) and acetic acid (480 mL). To the suspension at 16°C is added zinc dust (35.6 g, 544 mmol) (Note 5) portionwise over 5 min. After a period of 10 min, the cooling bath is removed to allow the internal temperature to reach 35°C. The mixture is stirred for about 3 hr until no hydrazide is detected by TLC (30% ethyl acetate in hexane, Rf = 0.3). The mixture is filtered over Celite and washed carefully with CH2Cl2 (15 × 40 mL). After removal of the solvents under reduced pressure, the brown mixture is dissolved in CH2Cl2 (800 mL) and saturated aqueous NaHCO3 solution (600 mL). Additional solid NaHCO3 is then added until pH 8 is attained. The resulting milky mixture is filtered over Celite, washed carefully with CH2Cl2 (10 × 100 mL), and the organic phase separated. The water phase is reextracted with CH2Cl2 (2 × 100 mL) and the combined organic layers are dried over sodium sulfate. After evaporation under reduced pressure, the resulting green residue is dissolved in hot CH2Cl2 (80 mL) and purified by flash chromatography (200 g of silica gel) (5% CH2Cl2/30% ethyl acetate/hexane). The indazole (14.02 g) is obtained as a yellowish solid. Crude product is recrystallized twice from ethyl acetate (100 mL) to provide 9.6 g of pure indazole (52%) as white crystals (Note 6). An additional 2.8 g (15%) of pure material is recovered from the mother liquor after recrystallization.
2. Notes
1.
3,5-Dimethoxyacetophenone 97%, anhydrous CH2Cl2, and trifluoromethanesulfonic acid were purchased from Aldrich Chemical Company, Inc., and used as received.
2.
Bis(2,2,2-trichloroethyl) azodicarboxylate was purchased from Aldrich Chemical Company, Inc. It can also be prepared as described in reference
2 3. See Hazard Index,
p. 837.
3.
The ground hydrazide was dried in order to remove trapped
ethyl acetate.
4.
An analytical sample was obtained by recrystallization from
ethyl acetate to afford a white solid, containing 0.5 equiv of
ethyl acetate, mp
137–139°C. The hydrazide exhibits the following spectral properties. IR (KBr) cm
−1: 3240, 1765, 1735, 1595;
1H NMR (400 MHz, acetone-d
6,325 K) δ: 2.65 (s, 3 H), 3.89 (s, 3 H), 3.90 (s, 3 H), 4.80 (s, 2 H), 4.90 (bs, 2 H), 6.76 (d, 1 H), 6.81 (d, 1 H), 8.91 (bs, 1 H);
13C NMR (100 MHz, acetone-d
6,325 K) δ: 56.19, 56.81, 75.44, 76.47, 96.00, 96.35, 102.39, 105.61, 121.48, 140.99, 154.57, 155.34, 158.03, 161.84, 200.40. Anal. Calcd for C
16H
16Cl
6N
2O
7·½ C
4H
8O
2: C, 35.70; H, 3.30; N, 4.62. Found C, 35.83; H, 3.14; N, 4.54. High-resolution mass spectrum, m/z calcd for C
16H
17Cl
6N
2O
7 (M+H)
+ 558.9167. Found 558.9166.
5.
Zinc dust was purchased from Anachemia. Excess
zinc was used to ensure completion of the reaction. The reactivity of the
zinc dust should be verified on a small scale first. Lower reactivity has been observed with another substrate using
zinc dust from Aldrich Chemical Company, Inc.6.
5,7-Dimethoxy-3-methylindazole has the following physical properties. mp
158°C. IR (KBr) cm
−1: 3315, 1600;
1H NMR (400 MHz, acetone-d
6) δ: 2.44 (s, 3 H), 3.82 (s, 3 H), 3.94 (s, 3 H), 6.45 (d, 1 H), 6.65 (d, 1 H);
13C NMR (100 MHz, CDCl
3) δ: 12.02, 55.47, 55.74, 90.84, 98.48, 123.41, 129.41, 142.64, 145.67, 155.26. Anal Calcd for C
10H
12N
2O
2: C, 62.49; H, 6.29; N, 14.57. Found C, 62.54; H, 6.32; N, 14.57. High-resolution mass spectrum, m/e calcd for C
10H
13N
2O
2 (M+H)
+ 193.0977. Found 193.0976.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The electrophilic, aromatic substitution of electron-rich arenes with the electron-deficient azodicarboxylate BTCEAD is a powerful method for the introduction, in a single operation, of a masked hydrazine or amino group.
4,5 Several activators can be used for this amination reaction;
ZnCl2,
5 BF3·Et2O,
5 LiClO4,
4 and here,
trifluoromethanesulfonic acid.
6 Trifluoromethanesulfonic acid dramatically increases the rate of the amination reaction and makes possible the use of this methodology with poorly reactive substrates.
The present, straightforward, two-step synthesis of 5,6-dimethoxyindazole from 3,5-dimethoxyacetophenone illustrates the usefulness of this amination reaction. With standard chemistry the introduction of a hydrazine group into the acetophenone molecule would have required four steps: 1) nitration, 2) reduction of the nitro group to the aniline, 3) diazotization and 4) reduction of the diazonium compound to the hydrazine.
A plausible mechanism for the formation of the indazole is illustrated below. Based on our previous observations that aryltrichlorohydrazides are readily converted to anilines, via the aryl hydrazines, it is unlikely that formation of the indazole ring 5 arises from the free hydrazine intermediate 2 since the corresponding aniline 6 was not detected at all in this case. This would suggest that the terminal trichloro ester group is selectively removed to give 3, followed by cyclization to 4 and cleavage of the second ester unit. Intermediate 4 was isolated and characterized during a probe reaction for which an equimolar amount of zinc dust was used. However, this intermediate does not accumulate to more than 1%. It appears that cleavage of the second ester group is relatively rapid after cyclization. Traces of compound 4 have also been detected during preparation of the hydrazide 1, presumably from acid hydrolysis.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
silica gel
3,5-DIMETHOXYACETOPHENONE
Aldrich Chemical Company, Inc
ZnCl2
LiClO4
acetic acid (64-19-7)
ethyl acetate (141-78-6)
sodium bicarbonate,
NaHCO3 (144-55-8)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
BF3·Et2O (109-63-7)
zinc (7440-66-6)
dichloromethane,
CH2Cl2 (75-09-2)
hexane (110-54-3)
trifluoroacetic acid (76-05-1)
trifluoromethanesulfonic acid (1493-13-6)
Bis(2,2,2-trichloroethyl) azodicarboxylate (38857-88-4)
5,7-Dimethoxy-3-methylindazole,
1H-Indazole, 5,7-dimethoxy-3-methyl- (154876-15-0)
1-(2-Acetyl-4,6-dimethoxyphenyl)-1,2-hydrazinedicarboxylic acid bis(2,2,2-trichloroethyl) ester
5,6-dimethoxyindazole
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved