Org. Synth. 1992, 70, 79
DOI: 10.15227/orgsyn.070.0079
PREPARATION AND DIELS-ALDER REACTION OF A REACTIVE, ELECTRON-DEFICIENT HETEROCYCLIC AZADIENE: DIMETHYL 1,2,4,5-TETRAZINE-3,6-DICARBOXYLATE. 1,2-DIAZINE (DIMETHYL 4-PHENYL-1,2-DIAZINE-3,6-DICARBOXYLATE) AND PYRROLE (DIMETHYL 3-PHENYLPYRROLE-2,5-DICARBOXYLATE) INTRODUCTION
[1,2,4,5-Tetrazine-3,6-dicarboxylic acid, dimethyl ester; 3,6-Pyridazinedicarboxylic acid, 4-phenyl-, dimethyl ester; 1H-Pyrrole-2,5-dicarboxylic acid, 3-phenyl-, dimethyl ester]
Submitted by Dale L. Boger, James S. Panek, and Mona Patel
1.
Checked by Richard Hutchings and Albert I. Meyers.
1. Procedure
A. Disodium dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate. A 2-L, three-necked, round-bottomed flask is equipped with an overhead stirrer, thermometer, and a 500-mL addition funnel. Sodium hydroxide (320 g, 8 mol) and 500 mL of water are added. Ethyl diazoacetate (200 g, 1.75 mol, (Note 1)) is placed in the addition funnel and added dropwise to the stirred sodium hydroxide solution so as to maintain the temperature of the reaction mixture between 60°C and 80°C (approximately 1.5 hr, (Note 2)). After the reaction slurry is cooled to room temperature, it is poured into 2 L of 95% ethanol, mixed well, and the liquid is decanted. This washing procedure is repeated five times using 1.5 L of 95% ethanol each time. The precipitate is collected by filtration using a Büchner funnel, the collected solid is washed with 1 L of absolute ethanol and 1 L of ether, and dried (12 hr) in the air to afford 160–184 g (85–97%) of disodium dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate as yellow brown solid.
B. Dihydro-1,2,4,5-tetrazine-3,6-dicarboxylic acid. A 2-L, three-necked, round-bottomed flask is equipped with an overhead stirrer and a 100-mL addition funnel. Disodium dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate (90.37 g 0.42 mol) in 100 mL of water and 100 g of crushed ice are added. The resulting slurry is cooled with an ice/sodium chloride bath and a solution of concentrated hydrochloric acid (84 mL of 36–38%) is added dropwise with stirring over 45 min. The reaction mixture is washed five times with 200 mL of dry ether and the ether layer is decanted. The product is immediately collected by filtration using a Büchner funnel. Drying the collected solid at room temperature under reduced pressure affords 51.6–53.06 g (72–74%) of dihydro-1,2,4,5-tetrazine-3,6-dicarboxylic acid as a yellow powder: mp 144–148°C (Note 3).
C. Dimethyl dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate. Absolute methanol (700 mL, (Note 4)) is placed in a dry, 2-L, round-bottomed flask fitted with an overhead stirrer, thermometer, and a 100-mL addition funnel and is cooled to −30°C. Thionyl chloride (62.12 g, 0.522 mol, 38.1 mL, (Note 5)) is added carefully with stirring and the reaction mixture is allowed to stir at −30°C for 30 min. Dihydro-1,2,4,5-tetrazine-3,6-dicarboxylic acid (45.00 g, 0.261 mol) is added as a solid in portions over 30 min to the cooled, stirred thionyl chloride-methanol solution (Note 6). The reaction mixture is allowed to warm to room temperature (1 hr) and is subsequently warmed to 35–40°C (internal temperature) for 2 hr. The reaction mixture is cooled to −30°C and the precipitate is collected by filtration using a Büchner funnel. The collected solid is washed with ether (115 mL, (Note 7)) and the filtrate is concentrated under reduced pressure to give an orange-brown oil (ca. 15 g). The collected solid is triturated with methylene chloride (2.0 L) and the insoluble inorganic salts are removed by filtration using a Büchner funnel. The orange-brown oil (ca. 15 g) is taken up in water (150 mL) and extracted with methylene chloride (6 × 270 ml). The combined methylene chloride extracts and methylene chloride triturate are dried over anhydrous sodium sulfate and concentrated under reduced pressure to afford 23–31 g (44–51%) of pure dimethyl dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate as an orange-yellow powder: mp 171–172°C (Note 8).
D. Dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate. Dimethyl dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate (20 g, 0.1 mol) is slurried in 800 mL of methylene chloride (Note 9) in a 2-L, round-bottomed flask fitted with a magnetic stirring bar and the mixture is cooled with an ice/water bath. A stream of nitrous gases (Note 10) is bubbled into the reaction mixture with stirring for 15 min. The color of the reaction mixture changes from orange to bright red during the addition. Stirring is continued for 1.5 hr as the reaction mixture is allowed to warm to room temperature. The solvent and excess nitrous gases are removed under reduced pressure to afford dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate (19.7 g, 99%) as a bright red, crystalline solid: mp 173–175°C (Note 11).
E. Dimethyl 4-phenyl-1,2-diazine-3,6-dicarboxylate. A 50-mL, round-bottomed flask equipped with a magnetic stirring bar is charged with dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate (1.0 g, 0.005 mol) and 1,4-dioxane (20 mL, (Note 12)). 1-Phenyl-1-(trimethylsiloxy)ethylene (1.07 g, 0.0056 mol, 1.14 mL, (Note 13)) is added and the reaction mixture is stirred under nitrogen at room temperature for 8 hr. The solvent is removed under reduced pressure to give a viscous oil that is triturated with anhydrous ether (2–3 mL). The solid product is collected by vacuum filtration and recrystallized from ethyl acetate/hexane to give 1.23–1.30 g (90–96%) of dimethyl 4-phenyl-1,2-diazine-3,6-dicarboxylate as a light yellow solid, mp 95.5–96°C (Note 14).
F. Dimethyl 3-phenylpyrrole-2,5-dicarboxylate. A 250-mL, round-bottomed flask equipped with a magnetic stirring bar is charged with dimethyl 4-phenyl-1,2-diazine-3,6-dicarboxylate (1.36 g, 0.005 mol) and glacial acetic acid (55 mL, (Note 15)). Zinc dust (3.25 g, 0.05 mol, (Note 16)) is added and the reaction mixture is stirred at room temperature for 6 hr. A second portion of zinc dust (3.25 g, 0.05 mol) is added and the reaction mixture is stirred for an additional 18 hr. The zinc dust is removed by filtration through a pad of Celite and the residue is washed with ether (100 mL). The filtrate and washes are combined, made basic (pH 10) with the addition of saturated sodium bicarbonate, and extracted with ether (2 × 100 mL). The combined ether extracts are dried over anhydrous magnesium sulfate and concentrated under reduced pressure. Purification of the product is effected by flash chromatography on a 4.5 × 9-cm column of silica gel (Aldrich 951, CH2Cl2 eluant), collecting 20-mL fractions. The fractions are analyzed by thin-layer chromatography (Kieselgel 60, CH2Cl2 eluant) and those containing product are combined and concentrated in vacuo to give 0.676 g (52%) of dimethyl 3-phenylpyrrole-2,5-dicarboxylate as a white solid: mp 122–123°C (ethyl acetate-hexane, (Note 17)).
2. Notes
1.
The submitters employed, without purification,
ethyl diazoacetate obtained from Aldrich Chemical Company, Inc.2.
A time lag (10–15 min) is observed before the exothermic reaction begins. Addition of
ethyl diazoacetate is then maintained at such a rate that the reaction temperature does not rise above 80°C. The checkers had to heat the mixture to 60°C.
3.
Drying of the free acid should be rapid with a large surface area since traces of
hydrochloric acid promote hydrolysis of the product to hydrazine salts. Slight warming (≤ 60°C) during drying accelerates the drying process. The IR spectrum is as follows: IR (KBr) γ
max cm
−1: 3700–3100, 3320, 3000–1850, 1710, 1630. The checkers found that this step does not work as well on a smaller scale (0.14 mol).
4.
Methanol is distilled from
magnesium turnings immediately before use.
5.
The submitters employed, without purification,
thionyl chloride obtained from Fisher Scientific Company. The procedure should be performed in a
well-ventilated hood since
thionyl chloride is a lachrymator. The yield of dimethyl ester was found to be lower in instances when the thionyl chloride-methanol solution was not allowed to stir (30 min, −30°C) prior to the addition of
dihydro-1,2,4,5-tetrazine-3,6-dicarboxylic acid.
6.
The temperature is maintained at −30°C during the additions.
7.
The submitters employed
ether distilled from
sodium benzophenone ketyl.
8.
The spectral properties of the product are as follows:
1H NMR (CDCl
3) δ: 3.92 (s, 6 H, CO
2CH
3), 7.42 (br s, 2 H, NH); IR (KBr) ν
max cm
−1: 3160, 3050, 1740, 1720.
9.
The submitters employed
methylene chloride from Fisher Scientific Company, which was distilled before use.
10.
Nitrous gases are generated in a separate vessel by the disproportionation of
nitrous acid (HONO):
200 mL of 6 N NaNO2 (1.2 mol) is added dropwise to
125 mL of concentrated hydrochloric acid (1.5 mol) in a
500-mL, three-necked, round-bottomed flask fitted with a nitrogen inlet, a 500-mL addition funnel, and an outlet tube leading to the reaction flask. The brown gases evolved are bubbled directly into the reaction mixture through a
5-mm (inside diameter) glass tube (smaller inlet tubes occasionally became plugged) using a
nitrogen stream.
CAUTION: all operations involving nitrous gases should be conducted in a well-ventilated hood because of the toxicity of these gases.11.
The checkers observed some starting material in the product which depressed the mp. It could be removed by crystallization from
ethyl acetate to give pure
3, mp
176–177°C, but with significant loss of product. The spectral properties of the product are as follows:
1H NMR (CDCl
3) δ: 4.22 (s, 6 H, CO
2CH
3); IR (KBr) η
max cm
−1: 2970, 1752, 1445, 1385, 1219, 1175, 1082, 960, 912; UV (dioxane) λ
max(log ε) 520 nm (2.754).
12.
The submitters employed
1,4-dioxane obtained from Fisher Scientific Company and distilled before use.
13.
The submitters employed, without purification,
1-phenyl-1-(trimethylsiloxy)ethylene obtained from Aldrich Chemical Company, Inc.14.
The elemental analysis and the spectral analysis of the product are as follows: Anal. Calcd for C
14H
12N
2O
4: C, 61.76; H, 4.44; N, 10.29. Found: C, 62.01; H, 4.50; N, 10.19;
1H NMR (CDCl
3) δ: 3.89 (s, 3 H, CO
2CH
3), 4.12 (s, 3 H, CO
2CH
3), 7.40–7.60 (m, 5 H, Ph), 8.27 (s, 1 H, C5-H); IR (KBr) ν
max cm
−1: 2955, 1742, 1584, 1447, 1399, 1287, 1244, 1142, 766; EI-MS (70 eV): m/e (relative intensity) 272 (M
+, 9), 242 (7), 241 (6), 214 (34), 182 (10), 155 (base), lit
3 mp
94–95.5°C.
15.
The submitters employed, without purification,
glacial acetic acid obtained from Fisher Scientific Company.
16.
Zinc dust obtained from Fisher Scientific Company was activated prior to use following an established procedure.
517.
The elemental analysis and the spectral analysis of the product are as follows: Anal. Calcd for C
14H
13NO
4: C, 64.86; H, 5.05; N, 5.40. Found: C, 65.10; H, 4.99; N, 5.48;
1H NMR (CDCl
3) δ: 3.82 (s, 3 H, OCH
3), 3.91 (s, 3 H, OCH
3), 6.94 (d, 1 H, J = 3, C4-H), 7.30–7.60 (m, 5 H, Ph), 9.80 (br, s, 1 H, NH); IR (KBr) ν
max cm
−1: 3314, 2958, 1726, 1564, 1464, 1436, 1270, 1096, 1008, 940, 846, 762, 696. The additional formation of
methyl 3-phenyl-5-carboxamidopyrrole-2-carboxylate in
32% yield is observed. The spectral properties of the product are as follows:
1H NMR (CDCl
3) δ: 1.75 (br s, 2 H), 3.92 (s, 3 H), 7.14 (s, 1 H), 7.40–7.60 (m, 5 H); IR (KBr) ν
maxcm
−1: 3442, 3346, 2950, 1708, 1642, 1580, 1528, 1476, 1366, 1280, 1132, 1022, 936, 808, 728, 618; CI-MS (70 eV): m/e (relative intensity) 245 (M
+ + H, base).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure describes the preparation and use of a reactive, electron-deficient heterocyclic azadiene suitable for Diels-Alder reactions with electron-rich, unactivated, and electron-deficient dienophiles.
6 7 8 9 10 11 Dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate, because of its electron-deficient character, is ideally suited for use in inverse electron demand (LUMO
diene-controlled)
12 Diels-Alder reactions. Table I and Table II detail representative examples of the reaction of
dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate with electron-rich
carbon dienophiles
13 and heterodienophiles,
6,7,8 respectively. Complete surveys of the reported Diels-Alder reactions of
dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate have been compiled.
6,7,8,9,10,11 Reductive ring contraction of the substituted
dimethyl 1,2-diazine-3,6-dicarboxylate [4 + 2] cycloadducts effected by
zinc in
acetic acid provides the corresponding substituted dimethyl pyrrole-2,5-dicarboxylates.
13,14 Table III details representative examples of this general reductive ring contraction reaction.
14,15
TABLE I
DIELS-ALDER REACTIONS OF DIMETHYL 1,2,4,5-TETRAZINE-3,6-DICARBOXYLATE: 1,2-DIAZINE INTRODUCTION13
|
|
Entry
|
|
Dienophile
|
Conditionsa Equiv. Temp °C (time hr)
|
1,2-Diazine
|
%Yield
|
|
|
|
1
|
|
|
1.5,25(12)
|
|
1
|
87
|
|
2
|
|
|
2-6,25(12)
|
1
|
trace
|
|
3
|
X = morpholine
|
|
2,25(48)
|
|
2
|
70
|
|
4
|
= pyrrolidine
|
2,25(48)
|
|
trace
|
|
5
|
|
|
1.5,25(12)
|
|
3
|
85
|
|
6
|
X = OSi(CH3)3
|
|
1,25(5)
|
|
4
|
92
|
|
7
|
= morpholine
|
1.2,25(1.5)
|
|
87
|
|
8
|
= pyrrolidine
|
1.5,25(12)
|
|
trace
|
|
9
|
|
|
1.5,25(0.5)
|
|
5
|
65
|
|
10
|
R = Si(Me)2-t-Bu
|
|
1.5,5-25(0.5)
|
|
6
|
33
|
|
11
|
|
|
2-3,25(6)
|
|
6
|
82
|
|
12
|
|
|
2.5,101(3)
|
|
7
|
71
|
|
13
|
|
1,25(5)
|
|
8
|
69
|
|
(a) All Diels-Alder reactions were carried out in dioxane.
|
TABLE II
IELS-ALDER REACTIONS OF DIMETHYL 1,2,4,5-TETRAZINE-3,6-DICARBOXYLATE WITH C=N HETERODIENOPHILES16 17 18
|
|
Dienophile
|
Conditions Temp °C (time hr)a
|
Product
|
% Yield
|
|
|
|
|
|
|
|
|
80(20)
|
68
|
|
|
80(8-12)
|
37
|
|
|
25(5)
|
-
|
|
|
25-50(25)
|
-
|
|
|
|
|
|
|
80(24)
|
65
|
|
|
60(10)
|
27
|
|
|
25(5)
|
-
|
|
|
|
|
|
80(4)
|
70
|
|
|
80(20)
|
33
|
|
|
80(4)
|
78
|
|
|
80(20-24)
|
82
|
|
(a) All Diels-Alder reactions were carried out in dioxane.
|
TABLE III
REDUCTIVE RING CONTRACTION OF SUBSTITUTED DIMETHYL 1,2-DIAZINE-3,6-DICARBOXYLATES: PYRROLE INTRODUCTION13
|
|
Entry
|
1,2-Diazine
|
Conditionsa Temp °C (time hr)
|
Pyrrole
|
%Yield
|
|
|
1
|
|
25(24)
|
|
63
|
|
2
|
|
25(24)
|
|
70
|
|
3
|
|
25(22)
|
|
52
|
|
4
|
|
25(9)
|
|
65
|
|
5
|
|
25(24)
|
|
67
|
|
6
|
|
25(24)
|
|
62
|
|
7
|
|
25(24)
|
|
56
|
|
8
|
|
25(36)
|
|
48
|
|
(a) All zinc (9-20 molar equiv) reductions were carried out in acetic acid (0.09 M in substrate).
|
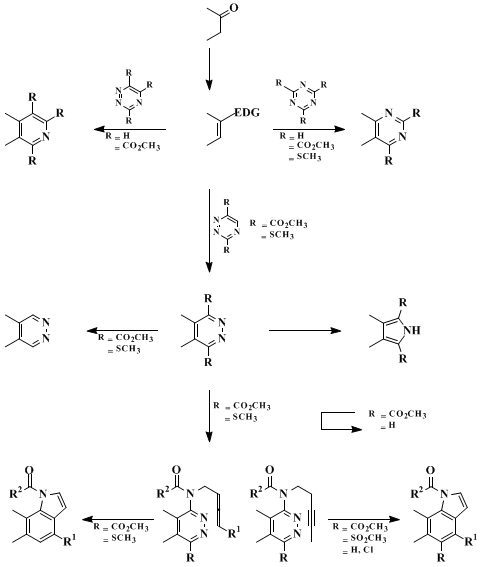
This approach to 1,2-diazine and pyrrole introduction based on the inverse electron demand Diels-Alder reaction of dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate complements the [4 + 2] cycloaddition reactions of a range of electron-deficient heterocyclic azadienes which permits the divergent preparation of a range of heterocyclic agents employing a common dienophile precursor, Scheme I.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
sodium benzophenone ketyl
1,2-DIAZINE (DIMETHYL 4-PHENYL-1,2-DIAZINE-3,6-DICARBOXYLATE)
PYRROLE (DIMETHYL 3-PHENYLPYRROLE-2,5-DICARBOXYLATE)
ethanol (64-17-5)
hydrochloric acid (7647-01-0)
acetic acid (64-19-7)
ethyl acetate (141-78-6)
methanol (67-56-1)
ether (60-29-7)
sodium hydroxide (1310-73-2)
thionyl chloride (7719-09-7)
sodium bicarbonate (144-55-8)
magnesium (7439-95-4)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
nitrous acid (7782-77-6)
carbon (7782-42-5)
zinc (7440-66-6)
methylene chloride (75-09-2)
Pyrrole (109-97-7)
magnesium sulfate (7487-88-9)
ethyl diazoacetate (623-73-4)
Dihydro-1,2,4,5-tetrazine-3,6-dicarboxylic acid (3787-09-5)
hexane (110-54-3)
1,4-dioxane (123-91-1)
1,2-DIAZINE (289-80-5)
Dimethyl 1,2,4,5-tetrazine-3,6-dicarboxylate,
1,2,4,5-Tetrazine-3,6-dicarboxylic acid, dimethyl ester (2166-14-5)
Dimethyl 4-phenyl-1,2-diazine-3,6-dicarboxylate,
3,6-Pyridazinedicarboxylic acid, 4-phenyl-, dimethyl ester (2166-27-0)
Dimethyl 3-phenylpyrrole-2,5-dicarboxylate,
1H-Pyrrole-2,5-dicarboxylic acid, 3-phenyl-, dimethyl ester (92144-12-2)
Disodium dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate (96898-32-7)
Dimethyl dihydro-1,2,4,5-tetrazine-3,6-dicarboxylate (3787-10-8)
1-Phenyl-1-(trimethylsiloxy)ethylene (13735-81-4)
methyl 3-phenyl-5-carboxamidopyrrole-2-carboxylate
dimethyl 1,2-diazine-3,6-dicarboxylate
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved