Org. Synth. 1997, 74, 137
DOI: 10.15227/orgsyn.074.0137
CYCLOPENTANONE ANNULATION VIA CYCLOPROPANONE DERIVATIVES: (3aβ,9bβ)-1,2,3a,4,5,9b-HEXAHYDRO-9b-HYDROXY-3a-METHYL-3H-BENZ[e]INDEN-3-ONE
Submitted by Michael J. Bradlee and Paul Helquist
1.
Checked by Steve Berberich and Stephen F. Martin.
1. Procedure
A. Magnesium enolate of 2-methyl-1-tetralone. Into a 500-mL, round-bottomed flask equipped with a magnetic stirring bar is placed 6.72 g of magnesium bromide diethyl etherate (MgBr2·Et2O) (26.0 mmol) under nitrogen (Note 1). Anhydrous ether, 350 mL, (Note 2) is added with a syringe, and the mixture is stirred at 25°C for a few minutes to dissolve the solid. Using an air-tight syringe, 11.2 mL of a 3.0 M solution of methylmagnesium bromide in ether (34 mmol, (Note 3)) is added to the slightly turbid solution at 25°C over a period of a few minutes. N,N-Diisopropylamine, 4.46 mL (34 mmol, (Note 4)) is added over 5 min, and the mixture is stirred for 16–18 hr, during which time a fine white precipitate forms (Note 5). The mixture is cooled to 0°C, and a solution of 5.28 g of 2-methyl-1-tetralone (33 mmol, (Note 6)) in 20 mL of anhydrous ether is added with a cannula over a 10-min period. The mixture becomes a bright yellow solution during the addition, and after 45 min of additional stirring, it becomes pale yellow.
B.
2-(1-Hydroxycyclopropyl)-2-methyl-1-tetralone. Simultaneously with the above operations, a separate
2-L, three-necked, round-bottomed flask is equipped with a
mechanical stirrer,
reflux condenser,
nitrogen inlet, and a
septum, and the flask is placed under a
nitrogen atmosphere. Anhydrous
ether, 600 mL, and
20 mL of a 3.0 M solution of methylmagnesium bromide in ether (60 mmol, (Note 3)) are added to the flask with a cannula and a syringe, respectively, and the solution is cooled to 0°C. A solution of
6.12 g of cyclopropanone ethyl hemiketal2 (60.0 mmol, (Note 7)) in 20 mL of anhydrous ether is added with vigorous stirring over several minutes with a cannula. During this addition, gas evolution is observed, and a fine white precipitate forms. After the mixture is stirred for 15 min, the solution of the enolate of
2-methyl-1-tetralone is added using a wide gauge cannula (ca. 1 mm id) over several minutes. The resulting turbid, light yellow solution is heated at reflux for 4 hr. The resulting clear, yellow solution is cooled to 25°C and diluted with
200 mL of ether. The organic solution is washed with saturated aqueous
ammonium chloride (3 × 200 mL) and saturated aqueous
sodium chloride (200 mL) and dried over anhydrous
magnesium sulfate. The drying agent is removed by filtration and the filtrate is concentrated by rotary evaporation under reduced pressure to give
7.92 g of crude
2-(1-hydroxycyclopropyl)-2-methyl-1-tetralone (Note 8) as a clear yellow oil that is used in the next step without further purification.
C.
(3aβ,9bβ)-1,2,3a,4,5,9b-Hexahydro-9b-hydroxy-3a-methyl-3H-benz[e]inden-3-one.3 The yellow oil from Part B is dissolved in
100 mL of anhydrous ether in a
100-mL round-bottomed flask at 25°C under
nitrogen. Into a separate 250-mL flask containing a magnetic stirring bar are placed
4.0 g of powdered sodium hydride (166 mmol, (Note 9)), and
100 mL of anhydrous ether at 25°C under
nitrogen. The solution of the yellow oil is added with stirring over a period of a few minutes using a cannula. The mixture is stirred at 25°C for 3 hr, during which time it becomes yellow-orange. The reaction is quenched by adding
100 mL of ice-cold saturated aqueous ammonium chloride and
100 mL of ether. The organic layer is washed with saturated aqueous
ammonium chloride (3 × 100 mL) and saturated aqueous
sodium chloride (100 mL), dried over anhydrous
magnesium sulfate, filtered, and concentrated by rotary evaporation under reduced pressure. The crude product is obtained as
5.8 g of yellow-orange crystals. The solid is dissolved in about
150 mL of warm ether, and
100 mL of hexanes is added. The mixture is concentrated to ca. 100 mL in a
heating bath (or on a steam bath), and the resulting cloudy solution is cooled slowly to 0°C. An initial crop of
4.73 g (
67%) of pale yellow crystals is obtained and the mother liquor is decanted from the solid and concentrated by rotary evaporation under reduced pressure to ca. 5 mL to give
0.39 g (
5%) of pale yellow crystals as a second crop. The mother liquor may then be concentrated and the residue purified by flash chromatography
4 on silica gel using 1:1
ether/
hexane to give
0.29 g (
4%) of additional product (R
f = 0.33) as yellow crystals after washing with warm 1:1
ether/
hexane. The total combined yield of
(3aβ,9bβ)-1,2,3a,4,5,9b-hexahydro-9b-hydroxy-3a-methyl-3H-benz[e]inden-3-one is
5.41 g (
76%)
(Note 10).
2. Notes
1.
The
MgBr2·Et2O was obtained from Aldrich Chemical Company, Inc., as a white solid in the form of a powder mixed with lumps. The solid was transferred to the
250-mL, round-bottomed flask in a nitrogen-filled glove box and carefully crushed with a
glass stirring rod until the solid was a uniform powder. The flask was then equipped with a septum, taken out of the glove box, and attached to a standard nitrogen/vacuum double manifold system to maintain an inert, dry atmosphere of prepurified
nitrogen in the flask throughout the remainder of the reaction sequence.
2.
Anhydrous
diethyl ether and anhydrous tetrahydrofuran (THF) were obtained from Fisher Scientific Company. They were redistilled under
nitrogen from dark blue or purple solutions of
sodium benzophenone ketyl immediately prior to use. All transfers of these and other anhydrous materials were performed with syringes or stainless steel cannulas while carefully maintaining a
nitrogen atmosphere.
3.
Methylmagnesium bromide was purchased from Aldrich Chemical Company, Inc., as a solution in
ether, and the solution was used as obtained without titration.
4.
N,N-Diisopropylamine was purchased from Aldrich Chemical Company, Inc., and distilled under
nitrogen from
calcium hydride prior to use.
5.
The submitters observed the fine white precipitate after 12 hr of stirring.
6.
2-Methyl-1-tetralone was purchased from Aldrich Chemical Company, Inc., and used without further purification.
7.
Cyclopropanone ethyl hemiketal is contaminated with 8–9% of the cyclopropanone methyl hemiketal as a result of exchange with the solvent.
2 The calculated number of mmoles of total cyclopropanone hemiketal is based upon this composition.
8.
1H NMR analysis of the crude product indicated greater than 90% conversion of the starting
2-methyl-1-tetralone to
2-(1-hydroxycyclopropyl)-2-methyl-1-tetralone: IR (CHCl
3) cm
−1: 3485 (O-H), 3067 (aromatic C-H), 2972 (aliphatic C-H), 1670 (C=O);
1H NMR (300 MHz, CDCl
3) δ:0.49–0.72 (m, 4 H, cyclopropyl CH
2CH
2), 1.20 (s, 3 H, CH
3), 1.61–1.71 (dt, 1 H, J = 13.4, 4.5, CHH), 2.03–2.15 (m, 1 H, CHH), 2.88–3.04 (m, 2 H, benzylic CH
2), 3.68 (s, 1 H, OH), 7.15–7.96 (m, 4 H, aromatic CH);
13C NMR (75 MHz, CDCl
3) δ: 9.0, 10.4 (cyclopropyl CH
2CH
2), 18.2 (CH
3), 25.5 (CH
2), 31.0 (benzylic CH
2), 48.0 (CC=O), 59.3 (cyclopropyl COH), 126.7, 127.8, 128.7, 131.7 (aromatic CH), 133.6, 143.4 (ipso aromatic C), 204.0 (C=O).
9.
Sodium hydride was obtained as a powder of 95% purity grade from Aldrich Chemical Company, Inc. A nitrogen-filled glove box was used to weigh the reagent and transfer it into the reaction flask.
10.
Yields of the product ranged from
67 to 81% over a series of several runs. For
(3aβ,9bβ)-1,2,3a,4,5,9b-hexahydro-9b-hydroxy-3a-methyl-3H-benz[e]inden-3-one, mp
117–118°C; IR (CHCl
3) cm
−1: 3466 (O-H), 3066, 3070 (aromatic C-H), 2976 (aliphatic C-H), 1736 (C=O);
1H NMR (300 MHz, CDCl
3) δ: 1.14 (s, 3 H, CH
3), 1.58–1.67 (m, 1 H), 1.80 (s, 1 H, OH), 1.87–1.99 (m, 1 H), 2.20–2.41 (m, 3 H), 2.48–2.61 (m, 1 H), 2.66–2.76 (m, 1 H), 2.84–2.97 (m, 1 H), 7.08–7.68 (m, 4 H, aromatic CH);
13C NMR (75 MHz, CDCl
3) δ: 14.5 (CH
3), 25.0 (CH
2), 29.1 (CH
2COH), 34.9 (CH
2C=O), 36.1 (benzylic CH
2), 52.5 (CCH
3), 79.8 (benzylic COH), 126.6, 126.9, 127.5, 128.5 (aromatic CH), 134.6, 140.5 (ipso aromatic C), 204.5 (C=O); CIMS, m/e (rel intensity) 217 (10%, MH
+), 199 (100%, MH
+ −18); Anal. Calcd for C
14H
16O
2: C, 77.75; H, 7.46. Found: C, 78.00; H, 7.60.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
A large number of methods have been developed for the construction of five-membered carbocyclic systems. These methods are too numerous to review here, but a number of key references are cited.
5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21The method that is used in the present preparation is based upon the formal concept of employing cyclopropanone in an aldol condensation with an enolate of another ketone. The resulting aldolate exhibits characteristics of a "homoenolate" and may undergo a second carbonyl addition reaction to form a five-membered ring as suggested in the following generalized pathway:
The homoenolate is not necessarily an intermediate in this pathway, but rather, it is shown only to illustrate the basic concept.
Cyclopropanone itself is a very unstable compound that has been isolated only at low temperature. Its chemistry has been studied thoroughly during the past several years.
22,23,24,25,26,27 Various forms of homoenolates have also been investigated, and their applications in synthesis are being actively developed.
28,29,30,31,32,33,34,35,36,37,38,39,40,41,42 The combination of
cyclopropanone and homoenolate chemistry employed here is most closely related to studies of Narasimhan
43,44 and follows from a series of studies in the laboratory of the submitters.
3,45,46,47,48,49 Whether free
cyclopropanone or the homoenolate form of the aldol adduct are produced as actual species in the above pathway is uncertain, but at least the formal concept of their hypothetical intermediacy and expected reactivity patterns have proven useful in the design of the annulation method employed in the present preparation. The
cyclopropanone hemiketal used in this work could conceivably undergo ethoxide elimination to produce
cyclopropanone in situ which would then serve as a very reactive acceptor for nucleophilic addition,
2,22,23,24,25,26,27,50 but other mechanistic pathways may also be consistent with the observed reaction sequence.
An attractive feature of this procedure is the directness with which the annulation of 3-hydroxycyclopentanone systems onto preexisting ketone skeletons can be accomplished to give usefully functionalized products. However, this method does have important limitations in its scope. The most common difficulty is that the "cyclopropanone" condensation with a wide range of ketone enolates often occurs in only low to modest yields. A two-fold excess of the
cyclopropanone hemiketal is therefore used to provide at least some improvement in the yields of this step. In contrast, the subsequent "homoenolate" cyclization generally occurs in quite acceptable yields. A simpler representative example that illustrates the contrasting yields in the two steps is the following:
46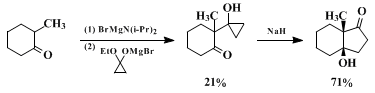
On the other hand, the parent, unsubstituted cyclohexanone enters into an apparently much more complex reaction pathway leading to the formation of a tricyclic, cycloheptanone-containing product.
45 Also, cycloheptanones as starting materials give annulation products that undergo a subsequent retro-aldol/re-aldol sequence to give rearranged hydrazulenones as the final isolated products. In fact, the best cases of the present
cyclopentanone annulation sequence are limited to benzo-fused cyclohexanones bearing an additional α-alkyl substituent. In addition to the preparation described here, another important example is the following (yields are not optimized as in the present preparation):
46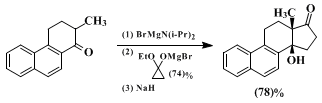
Although the limitations in the cases that provide good yields may appear to be very restrictive, this last example is suggestive of the potential applications of this procedure. Rather obvious possibilities that follow from this case include derivatives of the equilinin steroidal hormone system,
51 and of the steroidal cardiotonic agents, e.g., the cardenolides, digitoxigenin, bufadienolides, etc.
52
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
sodium benzophenone ketyl
magnesium bromide diethyl etherate
ether,
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
magnesium (7439-95-4)
sodium chloride (7647-14-5)
nitrogen (7727-37-9)
Cyclopentanone (120-92-3)
magnesium sulfate (7487-88-9)
methylmagnesium bromide (75-16-1)
Tetrahydrofuran (109-99-9)
sodium hydride (7646-69-7)
hexane (110-54-3)
calcium hydride (7789-78-8)
N,N-Diisopropylamine (108-18-9)
CYCLOPROPANONE (5009-27-8)
cyclopropanone ethyl hemiketal (13837-45-1)
cyclopropanone methyl hemiketal
cyclopropanone hemiketal
2-methyl-1-tetralone (1590-08-5)
2-(1-Hydroxycyclopropyl)-2-methyl-1-tetralone
(3aβ,9bβ)-1,2,3a,4,5,9b-hexahydro-9b-hydroxy-3a-methyl-3H-benz[e]inden-3-one
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved