Org. Synth. 1995, 72, 62
DOI: 10.15227/orgsyn.072.0062
SYNTHESIS OF (S)-2-METHYLPROLINE: A GENERAL METHOD FOR THE PREPARATION OF α-BRANCHED AMINO ACIDS
[L-Proline, 2-methyl-]
Submitted by A. K. Beck, S. Blank, K. Job, D. Seebach, and Th. Sommerfeld
1.
Checked by Frank Narjes and Larry E. Overman.
1. Procedure
A. (2R,5S)-2-tert-Butyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one. To a suspension of 40.0 g (0.347 mol) of (S)-proline (Note 1) in 1400 mL of pentane (Note 2) in a 2.5-L, round-bottomed flask are added 225 mL (2.072 mol) of pivalaldehyde (Note 3) and 3.0 mL (38.9 mmol) of trifluoroacetic acid. The mixture is heated at reflux for 72 hr with azeotropic removal of the water formed (Dean-Stark trap). After the addition of another 40.0 mL (0.368 mol) of pivalaldehyde, 1 mL of trifluoroacetic acid (13.0 mmol), and 200 mL of pentane (Note 4), refluxing is continued for an additional 72 hr. Water, 5.4 mL total, is collected. After the reaction mixture is cooled to room temperature, it is filtered under argon (Note 5). The resulting clear solution is concentrated in a 1000-mL flask under reduced pressure. The residue is distilled in a Kugelrohr oven (70°C/0.0005 mm) (Note 6) and (Note 7) to afford 42.5–47.0 g (0.232–0.257 mol, 67–74%) of the desired product as a colorless oil (Note 8) and (Note 9).
B. (2R,5S)-2-tert-Butyl-5-methyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one. In a 250-mL, round-bottomed flask equipped with a magnetic stirrer, 18.3 mL (0.131 mol) of diisopropylamine (Note 10) is mixed with 120 mL of dry tetrahydrofuran (THF, (Note 11)) under argon. At −78°C bath temperature, 88.6 mL of a 1.6 M solution of butyllithium (0.142 mol) in hexane is added and the mixture is allowed to warm to room temperature for 20 min. After the mixture is recooled to −78°C, the lithium diisopropylamide (LDA) solution is added over a period of 20 min (Note 12) to a solution of 20.0 g (0.109 mol) of (2R,5S)-2-tert-butyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one in 600 mL of dry THF in a 1-L, round-bottomed flask, precooled to −78°C. Tetrahydrofuran (20 mL) is used to rinse the 250-mL flask. After keeping the resulting solution at −78°C for 45 min, 8.8 mL (0.142 mol) of iodomethane (Note 13) is added over a period of 10 min. The resulting mixture is allowed to warm to 0°C over a period of 3 hr, and 300 mL of a saturated aqueous solution of ammonium chloride is added. After separation, the organic layer is washed with 300 mL each of saturated aqueous solutions of sodium carbonate and brine. Each aqueous layer is extracted twice with 200 mL of ethyl acetate. The combined organic layers are dried over magnesium sulfate and the solvent is removed in a rotary evaporator at ca. 15 mm. Traces of solvent are removed by drying the residue at 60°C/0.05 mm for 2 hr under an oil pump vacuum to yield 19.8–20.5 g (0.100–0.104 mol, 93–95%) of the desired product. It is used directly in the next step (Note 14).
C.
(S)-2-Methylproline. In a 1-L, round-bottomed flask,
20.1 g (0.102 mol) of (2R,5S)-2-tert-butyl-5-methyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one in
400 mL of 3 N hydrochloric acid (HCl) is heated to reflux for 1 hr. The water is removed under reduced pressure in a rotary evaporator (ca. 15 mm). The dark residue is treated with
400 mL of 3 N HCl and extracted four times with
200 mL each of dichloromethane (CH2Cl2). The combined organic layers are washed once with
200 mL of 3 N HCl. The combined aqueous layers are concentrated and dried under reduced pressure in a rotary evaporator at elevated temperature (60°C at 15 mm). The residue is suspended in 50 mL of water and adsorbed on
600 g of Dowex 50W × 8 (H+ form) (Note 15) in a
50 × 380-mm column. Water is passed through the column. After 200 mL of effluent, the pH changes to 2; after another 700 mL, the pH of the effluent is 7. The amino acid is then eluted with
3 N aqueous ammonia. After 500 mL of effluent, the developing hot front reaches the outlet. The following 2000 mL are collected and yield
12.0–12.3 g of the amino acid, free of inorganic salts, after removal of the water
(Note 16) and
(Note 17). The 500 mL of water collected subsequently contains another
0.34 g, yielding altogether
93–98 mmol [
85–90% from
(2R,5S)-2-tert-butyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one] of
(S)-2-methylproline,
[α]DRT −71.1° to −72.1° (MeOH,
c 1.0), mp
248–252°C (dec). The enantiomeric excess (ee) of a sample with a rotation of
−72.1° was shown to be 99.0 ± 0.5% by capillary gas chromatographic analysis of a derivative
(Note 18) using a chiral column
(Note 19) (see
Figure 1).
Figure 1. Gas chromatograms for the determination of the enantiomeric purity of the derivatives of 2-methylproline (Note 18) and (Note 19): A) (R)- and (S)-2-methylproline; B) (S)-2-methylproline; (C) (R)-2-methylproline; D) 1% (R)- in (S)-2-methylproline.
2. Notes
1.
(S)-Proline was used as commercially available. The submitters obtained
(S)-proline from Degussa AG (D-Hanau), while the checkers used material from Aldrich Chemical Company, Inc.2.
When higher boiling solvents such as
cyclohexane are used, the reaction is complete earlier, but side-products can be detected that are difficult to remove from the reaction mixture and that tend to catalyze decomposition of the desired product.
3.
Commercially available material can be used. As pure
pivalaldehyde is expensive, the submitters used technical grade as provided by BASF AG (D-Ludwigshafen). The material was washed with water (to remove alcohol impurities) and distilled before use. The checkers used material from Aldrich Chemical Company, Inc., without purification.
4.
Pentane has to be replenished since some is lost from the reaction mixture because of its low boiling point and the prolonged heating period.
5.
Since the product is extremely sensitive to hydrolysis, contact with air and moisture have to be avoided. Thus, the reaction mixture was filtered through a funnel under a stream of
argon.
6.
The use of an Aldrich Kugelrohr oven allowed for distillation in a large flask. This is advantageous, since decomposition may ensue above 100°C.
2 For this reason distillation has to be carried out at as low a pressure as possible. In most cases the submitters used a turbo pump, but application of a normal high
vacuum pump with a distillation temperature of 90°C at 0.04 mm is also possible.
7.
To recover part of the excess
pivalaldehyde, the material that was collected in the cooling trap used with the rotary evaporator and with the Kugelrohr oven can be distilled through a
35-cm Vigreux column; this yielded
150 mL of recovered pivalaldehyde.
8.
The spectrum is as follows:
1H NMR (200 MHz, CDCl
3) δ: 1.55–2.25 (m, 4 H), 2.98 [(m (centered), 2 H)], 3.78 (dd, 1 H, J = 9, 5), 4.49 (s, 1 H). Additional analytical data are given in ref.
3.
9.
As described in
(Note 5) above, the product hydrolyzes on contact with moisture (the clear liquid turns milky at the surface). Therefore any transfer of this compound must be carried out under
argon and the product must be stored under an inert atmosphere in a refrigerator.
10.
Diisopropylamine was distilled over
calcium hydride and stored under
argon before use.
11.
THF was freshly distilled under
argon over
potassium or
sodium/benzophenone.
12.
For addition of the
LDA solution, either a bowed metal needle or a Teflon cannula (2 mm diameter) can be used. See ref.
4 for a diagram of the set up.
13.
Iodomethane was filtered through neutral
aluminum oxide (the submitters used Alumina Woelm B, Akt. I) directly before use to remove moisture and decomposition products. (A layer of brown material was found on top of the
aluminum oxide when the
iodomethane had not been freshly distilled.)
14.
A pure sample for determining the analytical data of this bicyclic product was obtained by Kugelrohr distillation (bp
85°C at 0.05 mm). The spectrum is as follows:
1H NMR (200 MHz, CDCl
3) δ: 0.88 (s, 9 H), 1.36 (s, 3 H), 1.60–1.90 (m, 3 H), 2.10–2.25 (m, 1 H), 2.75–2.90 (m, 1 H), 3.05–3.20 (m, 1 H), 4.24 (s, 1 H). Further data for this compound are available in Ref.
3.
15.
Dowex 50W × 8 (Na+ form), as purchased from Fluka AG or recovered from previous use, is stirred for 30 min with ca.
6 N HCl, washed until nearly neutral, stirred with ca.
6 N ammonium hydroxide, washed with water, then stirred a second time with ca.
6 N HCl and washed until neutral (pH 7). The resin thus obtained is used directly.
16.
Though yellow-colored, the
2-methylproline thus obtained is spectroscopically pure (according to the
1H and
13C NMR spectra, see
(Note 17)). It can be further purified by dissolution in
methanol with 5% w/w activated charcoal and filtration of the resulting suspension through Celite. In spite of the poor crystallizing tendency of most amino acids,
2-methylproline can be recrystallized from
methanol/ethyl acetate to yield colorless platelets.
17.
The spectra are as follows:
1H NMR (200 MHz, D
2O, HDO = 4.80) δ: 1.52 (s, 3 H), 1.75–2.40 (m, 4 H), 3.20–3.45 (m, 2 H);
13C NMR (50 MHz, D
2O) δ: 23.99, 25.85, 38.27, 48.06, 73.21, 179.87.
18.
For gas chromatography, derivatives of the two enantiomers of the amino acid (the isopropyl amido isopropyl amides) were obtained according to the procedure developed by König:
5 6 7 In a
1-mL, sealed flask, 5 mg of the amino acid is treated with
0.3 mL of CH2Cl2 and
0.3 mL of isopropyl isocyanate at 100°C for 15 min. After the solution is cooled, both
CH2Cl2 and excess
isopropyl isocyanate are driven out by a strong stream of dry air. The residue is treated with
1 mL of diethyl ether and the resulting suspension is filtered through cotton wool. The solution can be used directly for determination of the ee by GC. This analysis was not checked by the checkers.
19.
The solution, 10 μL, prepared as outlined in
(Note 18) was loaded on a
Chirasil-Val fused-silica capillary column of Machery-Nagel (25 m, 0.4 mm) in a Carlo-Erba-Fraktovap 4160 HR GC. After 5 min at 160°C, the column temperature was increased by 2°C per min up to 200°C.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
rac-2-Methylproline has been obtained previously by Ellington and Honigberg
8 from
5-(3-hydroxypropyl)-5-methylhydantoin. For other preparations of enantiopure
2-methylproline see references
9 10 and
11. Methylation of
proline with retention of configuration as described here is an example of a general principle that has been applied to chiral α- and β-HX-substituted carboxylic acids (X = NH, O, S).
12 13 14 15 16 17 18 19,20 21 22,23 24 It involves a three-step sequence: diastereoselective conversion of the enantiopure carboxylic acid to a cyclic acetal derivative, diastereoselective replacement of one of the substituents on the original asymmetric carbon atom, and cleavage of the acetal. Since no chiral auxiliary molecule is employed in the procedure, in which the one and only chirality center of the starting material is temporarily eliminated (i.e., converted to a trigonal center), the overall transformation has been called self-regeneration of a stereogenic center,
25 26 27 as shown in the accompanying scheme.
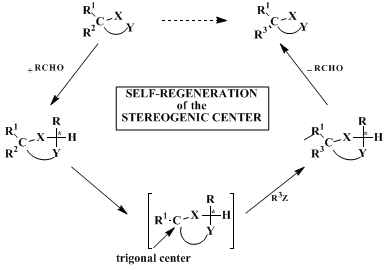
The intermediate trigonal center may be cationic, radical, anionic, or part of a double bond. By this methodology, the large supply of simple, mostly naturally occurring enantiopure compounds ("chiral pool"
28) can be used in many ways.
12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27α-Methylproline is an especially interesting α-branched amino acid for the synthesis of peptides and proteins that show an unusual stability to proteases.
29 Moreover incorporation of
α-methylproline into a peptide to replace
proline in a β-turn causes a significant increase in stability of this particular structural element.
30,31 32 33 34
Following exactly the same procedure, Parts A, B and C, but starting with (R)-proline gives (R)-2-methylproline, [α]DRT +73.1° (MeOH, c 1.7) in an overall yield of 55%.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
LDA
(R)- and (S)-2-methylproline
rac-2-Methylproline
hydrochloric acid,
HCl (7647-01-0)
ammonia (7664-41-7)
ethyl acetate (141-78-6)
methanol (67-56-1)
diethyl ether (60-29-7)
ammonium chloride (12125-02-9)
sodium carbonate (497-19-8)
cyclohexane (110-82-7)
Benzophenone (119-61-9)
sodium (13966-32-0)
ammonium hydroxide (1336-21-6)
potassium (7440-09-7)
iodomethane (74-88-4)
Pentane (109-66-0)
dichloromethane,
CH2Cl2 (75-09-2)
magnesium sulfate (7487-88-9)
aluminum oxide (1344-28-1)
butyllithium (109-72-8)
proline,
(S)-proline (147-85-3)
Tetrahydrofuran,
THF (109-99-9)
hexane (110-54-3)
argon (7440-37-1)
calcium hydride (7789-78-8)
trifluoroacetic acid (76-05-1)
lithium diisopropylamide (4111-54-0)
diisopropylamine (108-18-9)
pivalaldehyde (630-19-3)
isopropyl isocyanate (1795-48-8)
(R)-proline (344-25-2)
(S)-2-Methylproline,
2-methylproline,
(R)-2-methylproline,
L-Proline, 2-methyl- (42856-71-3)
(2R,5S)-2-tert-Butyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one (81286-82-0)
(2R,5S)-2-tert-Butyl-5-methyl-1-aza-3-oxabicyclo[3.3.0]octan-4-one (86046-11-9)
5-(3-hydroxypropyl)-5-methylhydantoin
α-Methylproline (475-11-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved