Org. Synth. 1992, 70, 157
DOI: 10.15227/orgsyn.070.0157
SUBSTITUTION REACTIONS OF 2-BENZENESULFONYL CYCLIC ETHERS: TETRAHYDRO-2-(PHENYLETHYNYL)-2H-PYRAN
[2H-Pyran, tetrahydro-2-(phenylethenyl)-]
Submitted by Dearg S. Brown and Steven V. Ley
1.
Checked by David J. Mathre and Ichiro Shinkai.
1. Procedure
An oven-dried, three-necked, 1-L, round-bottomed Morton flask equipped with a mechanical stirrer, gas bubbler outlet, 125-mL, pressure-equalizing addition funnel fitted with a rubber septum, and a nitrogen inlet (Note 1) is charged with 11.2 g (110 mmol) of phenylacetylene (Note 2) and 60 mL of dry tetrahydrofuran (THF) (Note 3). The addition funnel is charged with 60 mL of 2 M isopropylmagnesium chloride (Note 4) which is then added over a 5-min period (Note 5). The addition funnel is rinsed with 10 mL of dry THF and the solution is stirred at room temperature for 1 hr. The addition funnel is charged with 72 mL of 1 M anhydrous zinc bromide in THF (Note 6) which is then added to the light-grey solution over a 5-min period (Note 7). The addition funnel is rinsed with 10 mL of dry THF, and the mixture is stirred for a further 30 min at room temperature (Note 8). The addition funnel is charged with a solution of 22.6 g (100 mmol) of 2-(phenylsulfonyl)tetrahydro-2H-pyran (Note 2) dissolved in 100 mL of dry THF, which is then added over a 5-min period (Note 9). The resulting grey solution is stirred at room temperature for 18 hr and then quenched with 300 mL of 1 M hydrochloric acid (Note 10). The mixture is transferred to a single necked, 1-L, round-bottomed flask and concentrated under reduced pressure (40°C, 100 mm) to remove the THF. The residue is transferred to a 1-L separatory funnel and extracted with 300 mL of isopropyl acetate (i-PrOAc). The extract is sequentially washed with water (150 mL), 1 M aqueous dibasic potassium phosphate (3 × 150 mL), and brine (150 mL). The extract is dried over anhydrous sodium sulfate, filtered through a sintered glass funnel, washing the residue with more i-PrOAc, and then concentrated under reduced pressure (40°C, 10 mm) to give 19.6 g of crude product as a pale yellow liquid (Note 11). This is distilled under reduced pressure to afford 18.3 g (98%) of tetrahydro-2-(phenylethynyl)-2H-pyran as a colorless liquid, bp 110–115°C (0.01 mm) (Note 12).
2. Notes
1.
A constant stream of anhydrous
nitrogen (dried over molecular sieves) was maintained throughout the reaction.
2.
All the chemicals used in this procedure were purchased from Aldrich Chemical Company, Inc., and were used without further purification unless otherwise stated.
3.
Tetrahydrofuran was dried over 3Å molecular sieves (residual water content <20 μg/mL) and purged with
nitrogen prior to use.
4.
Isopropylmagnesium bromide can also be used in this type of experiment.
5.
The reaction is exothermic, the internal temperature rising from 20°C to 44°C.
CAUTION: Do not run the reaction under more concentrated conditions, or on a larger scale without providing external cooling.6.
Zinc bromide (100 g, 440 mmol) (98+%, (Note 2)) was dissolved in dry THF bringing the volume to 440 mL (exothermic heat of solution). The initial water content (1.2 mg/mL) was reduced to <50 μg/mL by drying the solution with 3Å molecular sieves (50 g) for 24 hr.
7.
The reaction is exothermic, the internal temperature rising from 20°C to 34°C. See
(Note 5).
8.
On addition of
zinc bromide a fine white precipitate is sometimes formed (observed by checkers).
9.
The reaction is exothermic, raising the internal temperature from 20°C to 32°C. See
(Note 5).
10.
The quench is exothermic during addition of the first ca.
25 mL of the aqueous hydrochloric acid, the internal temperature rising from 20°C to 30°C.
11.
HPLC analysis is as follows: 94.1 wt% product, 2.4 wt%
phenylacetylene, remainder i-PrOAc. HPLC conditions are as follows: [4.6 × 250 mm Zorbax RX; 40:60 H
2O (0.01 M KH
2PO
4)/MeCN; 1.5 mL/min; UV 210 nm]
phenylsulfinic acid (2.6 min),
2-(phenylsulfonyl)tetrahydro-2H-pyran (4.7 min, decomposes in solution),
phenylacetylene (6.1 min),
tetrahydro-2-(phenylethynyl)-2H-pyran (9.2 min).
12.
The physical properties are as follows: literature bp
149°C (8 mm);
2 HPLC analysis: >99 wt% product, <0.1%
phenylacetylene;
1H NMR (CDCl
3) δ: 1.50–2.00 (m, 6 H, C3-H
2, C4-H
2, C5-H
2), 3.53–3.66 (m, 1 H, C6-H), 4.00–4.13 (m, 1 H, C6-H), 4.52 (dd, 1 H, J = 2.8, 7.4, C2-H), 7.26–7.35 (m, 3 H, Ar-H), 7.41–7.51 (m, 2 H, Ar-H);
13C NMR (CDCl
3) δ: 21.8 (t), 25.7 (t), 32.2 (t), 66.6 (t), 67.5 (d), 85.2 (s), 88.1 (s), 122.8 (s), 128.2 (d), 128.3 (d), 131.8 (d).
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Methods for forming carbon-carbon bonds at the anomeric position of cyclic ethers are important processes in organic synthesis. We have shown how lactols and their derivatives can be readily converted into the corresponding 2-benzenesulfonyl cyclic ethers.
3,4 These versatile intermediates can then be transformed into the corresponding dihydropyrans,
3 2-substituted dihydropyrans,
4 spiroacetals,
4,5 and tetrahydropyranyl ethers
6 (Scheme 1).
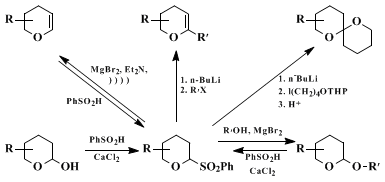
The procedure illustrated here is representative of a general and versatile method for the preparation of 2-substituted tetrahydrofurans and tetrahydropyrans from cyclic ether sulfones and the appropriate alkynyl, vinyl, or aryl Grignard reagent. From the examples shown in the Table and others previously reported,
3,7 a selectivity for the trans-product is observed with 6-substituted tetrahydropyrans irrespective of the initial geometry of the sulfone. This implies the presence of a common reaction intermediate such as an oxonium ion which is trapped by preferred axial bond formation at the 2-position. For 5-substitued tetrahydrofurans, significant trans-selectivity is only observed with large substituents.
TABLE
SUBSTITUTION REACTIONS OF 2-BENZENESULFONYL CYCLIC ETHERS
|
|
Sulfone
|
Product(s)
|
Yield (%)
|
|
|
|
|
83
|
|
|
|
81
|
|
|
|
91
|
|
|
|
95
|
|
|
|
82
|
|
|
|
54
|
|
The benzenesulfones also undergo nucleophilic displacement with
silyl enol ethers and
ketene silyl acetals in the presence of a Lewis acid such as
aluminum trichloride (Scheme 2).
8
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
brine
hydrochloric acid (7647-01-0)
sodium sulfate (7757-82-6)
nitrogen (7727-37-9)
aluminum trichloride (3495-54-3)
Ketene (463-51-4)
Phenylacetylene (536-74-3)
isopropylmagnesium bromide (920-39-8)
isopropylmagnesium chloride (1068-55-9)
Tetrahydrofuran (109-99-9)
zinc bromide (7699-45-8)
isopropyl acetate (108-21-4)
phenylsulfinic acid (618-41-7)
silyl (13765-44-1)
Tetrahydro-2-(phenylethynyl)-2H-pyran (70141-82-1)
2H-Pyran, tetrahydro-2-(phenylethenyl)-
2-(phenylsulfonyl)tetrahydro-2H-pyran (96754-03-9)
potassium phosphate (7778-53-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved