Org. Synth. 1998, 75, 153
DOI: 10.15227/orgsyn.075.0153
WITTIG OLEFINATION OF PERFLUOROALKYL CARBOXYLIC ESTERS; SYNTHESIS OF 1,1,1-TRIFLUORO-2-ETHOXY-5-PHENYLPENT-2-ENE AND 1-PERFLUOROALKYL EPOXY ETHERS: 1,1,1-TRIFLUORO-2-ETHOXY-2,3-EPOXY-5-PHENYLPENTANE
[
(Benzene, (4-ethoxy-5,5,5-trifluoro-3-pentenyl)-, (Z)- and Oxirane, 2-ethoxy-3-(2-phenylethyl)-2-(trifluoromethyl)-, cis-(±)-)
]
Submitted by Jean-Pierre Bégué, Danièle Bonnet-Delpon, and Andrei Kornilov
1
.
Checked by Yugang Liu and Robert K. Boeckman, Jr..
1. Procedure
A. (Z)-1,1,1-Trifluoro-2-ethoxy-5-phenyl-2-pentene
(3). A dry, 250-mL, three-necked, round-bottomed flask
(Note 1), equipped for magnetic stirring and with a rubber septum, argon inlets, and calcium chloride drying tube, is flushed with dry argon
(Note 2) and charged with
2.31 g (77 mmol) of an 80% suspension of sodium hydride (NaH) in mineral oil (Note 3). The suspension is washed free of oil with two portions of dry
pentane
. The flask is then charged with
100 mL of tetrahydrofuran
(Note 4), and
32.3 g (70 mmol) of 3-phenylpropyltriphenylphosphonium bromide
(1)
(Note 5), and equipped with a condenser bearing the calcium chloride drying tube. The reaction mixture is stirred under reflux for 5 hr and then cooled to ~ 0°C with an ice bath (Note 6). By syringe,
10.0 g (70 mmol) of ethyl trifluoroacetate
(2)
is slowly added through the septum inlet to the stirred reaction mixture (Note 7). After the addition is complete, the ice bath is removed and the reaction mixture is heated at reflux for 1 hr, cooled, and diluted with
300 mL of pentane
. The supernatant liquid from the resulting suspension is decanted and filtered through a short silica gel column
(Note 8). The residual solids are washed twice with
50 mL of pentane
, and the combined pentane solutions are passed through the same column. The column is then eluted with
200 mL of a 5:1 (v/v) mixture of pentane-diethyl ether
. Evaporation of the solvents from the combined eluates under reduced pressure and bulb-to-bulb vacuum distillation of the residual liquid (oven temperature 120-130°C at 10 mm) provides 12.0-12.8 g (70-75%) of 97% pure (by gas chromatography) (Z)-1,1,1-trifluoro-2-ethoxy-5-phenyl-2-pentene (3)
as a clear, colorless liquid, bp 80°C (10 mm)
(Note 9).
B. 1,1,1-Trifluoro-2-ethoxy-2,3-epoxy-5-phenylpentane
(4). A 250-mL, round-bottomed flask, equipped for magnetic stirring and with a condenser fitted with a calcium chloride drying tube, is charged with
9.76 g (40 mmol) of 1,1,1-trifluoro-2-ethoxy-5-phenyl-2-pentene
(3)
, and a solution of
14.79 g (60 mmol) of 70% meta-chloroperoxybenzoic acid
in
170 mL of dichloromethane
(Note 10) and (Note 11). The resulting mixture is heated under reflux with stirring for 20 hr (Note 12), cooled, and concentrated to 50-60 mL under reduced pressure. The residual liquid is diluted with
200 mL of pentane
and the supernatant liquid from the resulting suspension is passed through a short silica gel column (Note 8). The residual solids are washed twice with
50 mL of a 10:1 mixture (v/v) of pentane-diethyl ether
, and the wash solutions are passed through the same column. The column is then eluted with
100 mL of a 5:1 (v/v) mixture of pentane-diethyl ether
. The combined eluants are concentrated under reduced pressure and the residual liquid is purified by bulb-to-bulb vacuum distillation (oven temperature 120-130°C at 10 mm) to provide 9.36-9.88 g (90-95%) of pure
1,1,1-trifluoro-2-ethoxy-2,3-epoxy-5-phenylpentane
(4)
as a clear, colorless liquid, bp 90°C (10 mm)
(Note 13).
2. Notes
1.
All of the glassware used in the preparation of enol ether
(3) was dried for at least 10 min at 200°C, assembled hot, and allowed to cool under an atmosphere of
argon.
2.
A slight positive pressure of
argon is maintained throughout the reaction.
3.
An
80% suspension of NaH was obtained from Alfa Inorganics Inc.
4.
Tetrahydrofuran was distilled from
sodium benzophenone ketyl
immediately before use.
5.
3-Phenylpropyltriphenylphosphonium bromide
(1) was prepared using the following procedure: A solution of 1 equiv of
1-bromo-3-phenylpropane and 1.05 equiv of triphenylphosphine (both obtained from Aldrich Chemical Company, Inc.) in dry
toluene
is heated at reflux for 50 hr. The resulting solids are collected by vacuum filtration, washed on the filter three times with dry
pentane, and dried at 100°C/1 mm for 6 hr affording
1 in
87-92%yield.
6.
The color of the reaction mixture became yellow after 10 min of reflux, and then orange after 2-3 hr. A catalytic amount of
hexamethyldisilane
can be added to accelerate the formation of the phosphorane.
7.
Ethyl trifluoroacetate
(2), obtained from Aldrich Chemical Company, Inc.
, is distilled and stored over
sodium bicarbonate
before use.
8.
A
50-g portion of silica gel 60 (70-200 microns) was used for this procedure.
9.
1,1,1-Trifluoro-2-ethoxy-5-phenyl-2-pentene
(3) displays the following spectral properties: IR (neat) cm
−1: 1670 (νC=C)
;
19F NMR δ: −68.0
;
1H NMR (300 MHz) δ: 1.30 (t, 3 H, J = 7), 2.53 (m, 2 H), 2.75 (t, 2 H, J = 7.3), 3.81 (q, 2 H, J = 7), 5.70 (t, 1 H, J = 7), 7.20-7.35 (m, 5 H)
;
13C NMR δ: 15.1, 26.5, 34.7, 69.4, 121.3 (q,
1J = 275), 126.0, 128.2 (2C), 128.5, 140.7, 143.1 (q,
2J = 32)
.
2
10.
Reagent grade
dichloromethane obtained from J. T. Baker Inc.
was used.
11.
A solution of
14.79 g of crude commercial meta-chloroperoxybenzoic acid (Aldrich Chemical Company, Inc., or Janssen Chimica, approximately 70%) in
150 mL of dichloromethane
is dried over
magnesium sulfate (MgSO4), filtered, and the MgSO
4 is washed twice with
10 mL of dichloromethane
.
12.
The process was monitored by GC analysis (SGE 25QC3, BPX5, 25 m × 0.32μ capillary column, 0.25μ film thickness); the retention time was 10.4 min for the enol ether
(3) and 1.1 min for the epoxide
(4).
13.
1,1,1-Trifluoro-2-ethoxy-2,3-epoxy-5-phenylpentane
(4) displays the following spectral properties:
19F NMR δ: −76.6
;
1H NMR (300 MHz) δ: 1.32 (t, 3 H, J = 7, CH
3), 2.07 (m, 2 H), 2.88 (m, 2 H), 3.36 (t, 1 H, J = 6.1), 3.80 (m, 2 H), 7.28 (m, 3 H), 7.39 (m, 2 H)
;
13C NMR (75 MHz) δ: 14.9, 28.2, 31.6, 61.0, 63.5, 81.5 (q,
2J
CF = 27.2), 122.0 (q,
1J
CF = 272), 126.0, 128.1, 128.3, 140.3
.
3
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure described here illustrates a general and inexpensive two-step method for the stereoselective preparation of new, variously substituted 1-CF
3 epoxy ethers from
ethyl trifluoroacetate.
4,2,3 The first step of this procedure is a Wittig olefination of
ethyl trifluoroacetate in which
sodium hydride is used for the generation of the ylide in order to obtain the best yield of desired enol ether
(3). The base used to prepare the phosphorane strongly influences the nature and yield of the resulting products.
2 Enol ethers
(3) with different substituents were obtained by this procedure. The reaction is successful with other alkyl perfluorinated alkanoates, where R
F can be different perfluoroalkyl substituents (C
2F
5, n-C
3F
7, etc.), R
1 can be a variety of aryl or alkyl substituents, and R
2 can be various primary alkyl or silyl groups.
4,2 All enol ethers are colorless liquids, stable to distillation under reduced pressure and storage in the refrigerator. 1-Perfluoroalkyl enol ethers
(3) have been used in the preparation of homoallylic fluorinated ketones,
5 vinyl bromides,
6 and trisubstituted trifluoromethyl alkenes.
6
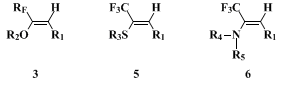
Corresponding vinyl sulfides
(5)
7 and enamines
(6)
8 are accessible by the same Wittig reaction with alkyl thiotrifluoroacetates and disubstituted-trifluoroacetamides respectively.
The second step of the procedure reported here is the usual epoxidation by
meta-chloroperoxybenzoic acid. We have obtained the cis-epoxides
(4) with different perfluoroalkyl (R
F) and aryl or alkyl (R) substituents:
3
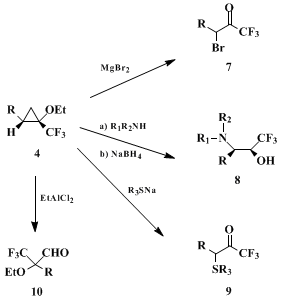
Recently 1-perfluoroalkyl epoxy ethers have been useful starting synthons for the preparation of various perfluoroalkylated organic compounds. For example, in the reaction with
magnesium bromide (MgBr2), α-bromoalkyl perfluoroalkyl ketones
7 were obtained;
3 reaction with secondary amines led to α-amino ketones and β-amino alcohols
8;
9,10 and treatment with sodium thiolates provided α-thioalkyl trifluoromethyl ketones
9.
11 Chlorohydrins, α-alkoxy aldehydes
10 and 2-hydroxytetralins could be prepared by the treatment with Lewis acids.
12
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
1,1,1-Trifluoro-2-ethoxy-2,3-epoxy-5-phenylpentane:
Oxirane, 2-ethoxy-3-(2-phenylethyl)-2-(trifluoromethyl)-, cis-(±)- (13);
(141937-91-9)
(Z)-1,1,1-Trifluoro-2-ethoxy-5-phenyl-2-pentene:
Benzene, (4-ethoxy-5,5,5-trifluoro-3-pentenyl)-, (Z)- (13);
(141708-71-6)
Sodium hydride (8,9);
(7646-69-7)
3-Phenylpropyltriphenylphosphonium bromide:
Phosphonium, triphenyl(3-phenylpropyl)-, bromide (8 ,9);
(7484-37-9)
Ethyl trifluoroacetate:
Acetic acid, trifluoro-, ethyl ester (8,9);
(383-63-1)
m-Chloroperoxybenzoic acid:
Peroxybenzoic acid, m-chloro- (8);
Benzocarboperoxoic acid, 3-chloro- (9);
(937-14-4)
1-Bromo-3-phenylpropane:
Benzene, (3-bromopropyl)- (8,9);
(637-59-2)
Triphenylphosphine:
Phosphine, triphenyl- (8,9);
(603-35-0)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved