Org. Synth. 1999, 76, 12
DOI: 10.15227/orgsyn.076.0012
(4R,5R)-2,2-DIMETHYL-α,α,α',α'-TETRA(NAPHTH-2-YL)-1,3-DIOXOLANE-4,5-DIMETHANOL FROM DIMETHYL TARTRATE AND 2-NAPHTHYL-MAGNESIUM BROMIDE
[
1,3-Dioxolane-4,5-dimethanol, 2,2-dimethyl-α,α,α',α'-tetra-2-naphthalenyl-, (4R-trans)-
]
Submitted by Albert K. Beck
1
, Peter Gysi
2
, Luigi La Vecchia
2
, and Dieter Seebach
1
.
Checked by Chad E. Bennett and William R. Roush.
1. Procedure
A. (R,R)-Dimethyl O,O-isopropylidenetartrate. Under an inert atmosphere (Note 1) a 2-L, two-necked, round-bottomed flask, equipped with a magnetic stirring bar and a pressure-equalized addition funnel, is charged with
(R,R)-dimethyl tartrate (89.1 g, 0.5 mol)
(Note 2) dissolved in
acetone (900 mL). To the clear solution is added, at room temperature,
boron trifluoride diethyl etherate (82.5 mL
48% solution, 0.31 mol)
(Note 3) over 30–40 min (Note 4). The resulting yellow solution is stirred for an additional 3 hr during which time the color of the solution becomes red-brown.
For workup the reaction mixture is poured into an aqueous saturated
sodium bicarbonate solution (4 L)
(Note 5). The turbid mixture is divided into two parts, and each part is extracted three times with
ethyl acetate (3 × 500 mL). The combined organic layers are washed twice with water (2 × 1 L) and dried over anhydrous
magnesium sulfate
. After filtration the solvent is removed by rotary evaporation at ca. 45°C/20 mm. The yellow oil that is obtained (103 g) is purified by fractional distillation using a 15-cm Vigreux column (bp 92–95°C/1.5 mm) to afford 84.3 g (77%) of product as a yellowish oil with a specific rotation of [α]RT
D
−62.0° (neat), −44.0° (CHCl3, c 1)
(Note 6) and (Note 7).
B. 2-Naphthylmagnesium bromide. Under an inert atmosphere (Note 1) a 4-L, four-necked, round-bottomed flask, fitted with a reflux condenser, pressure-equalized addition funnel, mechanical stirring bar and a thermometer, is charged with
magnesium turnings (24.7 g, 1.02 at. equiv) and some
iodine
crystals. Then
30 mL of a solution of 2-bromonaphthalene (200.7 g, 0.97 mol) (Note 8) in tetrahydrofuran (THF) (675 mL)
(Note 9) is added. As soon as the reaction has started (Note 10), the remainder of the tetrahydrofuran solution is added at such a rate that a gentle reflux is maintained (Note 11). After complete addition, reflux is continued for 1 hr by heating with an oil bath
(Note 12). Finally, the reaction mixture is allowed to cool to room temperature.
C.
(4R,5R)-2,2-Dimethyl-α,α,α',α'-tetra(naphth-2-yl)-1,3-dioxolane-4,5-dimethanol
. To the Grignard solution obtained in Part B is added, with stirring and cooling by an ice bath, a solution of
(R,R)-dimethyl O,O-isopropylidenetartrate (48.1 g, 0.22 mol) (see Part A) in
tetrahydrofuran (480 mL). During the addition the internal temperature should not exceed 20°C (Note 13) and (Note 14). After completion of the addition, the reaction mixture is heated at reflux for 1.5 hr using an oil bath, then cooled to room temperature.
For workup, an aqueous saturated
ammonium chloride solution (1.6 L) is carefully added, cooling the mixture with an ice bath (Note 15) and (Note 16). The mixture is extracted once with
ethyl acetate (500 mL)
(Note 17). After separation of the layers, the aqueous phase is extracted twice with
ethyl acetate (2 × 250 mL). The combined organic layers are washed twice with
brine (2 × 250 mL) and dried over anhydrous magnesium sulfate, and the solvent is evaporated on a rotary evaporator at 45°C/100 mm. The resulting yellowish foam (200 g) (Note 18) and (Note 19) is dissolved in
diethyl ether (100 mL) followed by the addition of
ethanol (400 mL). After a few minutes a white solid precipitates that is the clathrate of the product with ethanol
(Note 20). After standing for several hours (or overnight), the crystals are filtered, washed with
ethanol/diethyl ether (300 mL, 4:1), and
ethanol (100 mL), and then dried overnight at 50°C/8 mm to give 125–130 g of colorless crystals (Note 21).
In order to remove the ethanol, the crystals are dissolved in
toluene (3 mL per g of clathrate) at 70°C, and the solution is evaporated to dryness on a rotary evaporator at 45°C/100 mm. This procedure is repeated once more. A portion of the solid so obtained (68.5 g) is mixed with
toluene (800 mL) at 80°C in a 2-L, two-necked, round-bottomed flask equipped with an overhead, mechanical stirrer, until the TADDOL [(R,R)-2,2-dimethyl-α,α,α',α'-tetra(naphth-2-yl)-1,3-dioxolane-4',5'-dimethanol] is completely dissolved.
Hexane (800 mL) is added slowly with the solution being maintained between 65–70°C. On cooling, a white precipiate starts to form at approximately 58°C. The mixture is allowed to cool completely to room temperature, and the resulting thick slurry is stirred overnight. Further solvent (
500 mL of a 1 : 1 mixture of toluene-hexane
) is added to the now unstirrable suspension. The mixture is shaken vigorously to give a stirrable slurry. The solid is removed by vacuum filtration and washed first with the mother liquor, then with a 1 : 1
toluene/hexane mixture (300 mL), and finally with
hexane (300 mL). The resulting solid is dried under high vacuum (0.3 mbar) at 90°C for 10 hr in a vacuum oven to give the title TADDOL ligand as a white solid (42.0 g, 61%). The mother liquor is concentrated under vacuum to give a yellow solid (24.5 g) that is again purified as described above by recrystallization from
300 mL of toluene
and
300 mL of hexane
to give a second crop of the TADDOL ligand (14.5 g, 21%), for a combined yield of 56.5 g (82%), mp 204–208°C (sintering at 155°C), [α]RT
D
−115.4° (ethyl acetate, c 1) (Note 22), (Note 23).
2. Notes
1.
The reaction can be carried out either under an
argon atmosphere, using a balloon, or under
nitrogen, passing a continuous flow of
nitrogen over the solution.
2.
(R,R)-Dimethyl tartrate is commercially available. The submitters used product donated by the Chemische Fabrik Uetikon, Uetikon, Switzerland, whereas the checkers used material obtained from Lancaster.
3.
Boron trifluoride-diethyl etherate solution, 48%
, is commercially available and was used without prior purification. Both the submitters and checkers used reagent grade material from Fluka Chemie AG.
4.
Boron trifluoride-diethyl etherate should be added at such a rate that the internal temperature does not exceed 30°C.
5.
Caution! Vigorous evolution of
carbon dioxide
takes place, which causes foaming. Use an appropriately sized beaker (10 L).
6.
The product has the following spectral properties:
1H NMR (200 MHz, CDCl
3) δ: 1.50 (s, 2 OCH
3), 3.85 (s, O-C(CH
3)
2-O), 4.80 (s, 2 H-C-O)
;
13C NMR (100 MHz, CDCl
3) δ: 26.2, 52.7, 76.9, 113.8, 170.0
; IR (neat) cm
−1: 2992, 2957, 1761
. For comparison with literature data see Refs.
3 and
4
5
6
7
7.
For an alternative synthesis using
tartaric acid
and
2,2-dimethoxypropane
in
methanol
see Ref.
4
5
6
7.
8.
2-Bromonaphthalene is commercially available or can be prepared according to an Organic Syntheses Procedure, see Ref.
8
9.
The submitters used
tetrahydrofuran p.a. quality.
10.
Usually warming with a heat gun is necessary to start the reaction.
11.
The addition takes about 45 min.
12.
After completion of the reaction some
magnesium particles remain (an ca. 5% excess was employed).
13.
The addition usually takes about 20 min.
14.
It is important to keep the internal temperature below 20°C; otherwise the yield decreases. Efficient cooling is therefore necessary.
15.
At the beginning, the addition of the aqueous saturated
ammonium chloride solution is highly exothermic and efficient cooling is required. During the addition, precipitation of magnesium salts takes place but they dissolve as addition progresses.
16.
At the end of the addition the reaction mixture should have a pH of 7–8; if not,
hydrochloric acid (HCl) 10% aqueous solution (the checkers needed 600 mL) should be added to reach this pH range.
17.
The submitters removed most of the
tetrahydrofuran using a rotary evaporator at 45°C/100 mm before extracting the residue with either isopropyl or
ethyl acetate. However, the checkers experienced significant bumping of the solution during the rotary evaporation step, and found it advantageous simply to subject the entire aqueous
THF mixture to the extraction procedure.
18.
Caution! Towards the end, the evaporation apparatus must be watched carefully because of foaming (adjust the pressure).
19.
Small samples of the crude product can also be purified by flash chromatography using
toluene as eluent, product R
f = 0.1 (
toluene).
20.
It is well known that TADDOLs easily form clathrates, (see Ref.
9
10
11
12).
21.
The clathrate obtained contained 1 equiv of
ethanol, as shown by
1H NMR spectroscopy. However, the submitters obtained 158 g of clathrate that contained 2 equiv of
ethanol per mol of TADDOL. For the crystal structure of the clathrate of this TADDOL with
piperidine
see Ref.
13
22.
The checkers obtained a
73% yield when following this crystallization procedure using crude, unpurified clathrate, and an
89% yield when once crystallized
TADDOL was recrystallized using this procedure.
23.
The product has the following spectral properties:
1H NMR (200 MHz, CDCl
3) δ: 1.18 (s, 2 CH
3), 4.22 (s, 2 OH), 4.98 (s, 2 H-C-O), 7.21–8.19 (m, arom. H)
;
13C NMR (100 MHz, CDCl
3) δ: 27.4, 78.6, 81.4, 109.9, 125.8, 125.89, 125.92, 126.0, 126.09, 126.13, 126.8, 127.1, 127.2, 127.3, 127.4, 127.9, 128.5, 128.6, 132.5, 132.62, 132.63, 132.7, 140.5, 142.5
. For comparison with literature data see Ref.
3
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
The procedure described here is a typical one for the preparation of α,α,α',α'-tetraaryl 2,2-disubstituted 1,3-dioxolane-4,5-dimethanols (TADDOLs,
1), a class of diols of which ca. 50 representatives have been synthesized.
13 They have become useful chiral auxiliaries for the preparation of enantiomerically enriched or pure compounds and for analytical purposes. The diols themselves have been employed as clathrate-forming compounds (resolutions and solid-state reactions
9,10,11,12) and NMR shift reagents.
12,14 The main applications involve metal complexes such as the Ti-TADDOLates
2 and their equally readily available enantiomers ent-
2. The substituents X and Y on Ti may be OR, Cl, Br, or
cyclopentadienyl. The reactions for which these chiral Lewis acids have been used in equimolar or catalytic amounts include: nucleophilic additions (of alkyl, aryl, or allyl groups) to aldehydes, ketones and nitroolefins, aldol additions, hydrophosphonylations, cyanohydrin reactions, intra-, intermolecular and hetero Diels-Alder additions, [2+2]- and [3+2]-cycloadditions, intra- and intermolecular ene reactions, iodolactonizations, transesterifications, and anhydride alcoholyses. TADDOL derivatives have also been applied in enantioselective lithium aluminium hydride reductions, Michael additions of Li enolates, hydrosilylations, Pd-catalyzed allylations and metatheses. Polymer-bound TADDOLs have also been used, and review articles with leading references have been published recently.
15
16
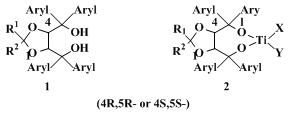
In many instances, the tetranaphthyl-substituted diol, the preparation of which is described here, gave the most effective chiral
titanium
catalysts. Three examples are the nucleophilic addition of
diethylzinc
to aldehydes (eq 1),
17 the Diels-Alder reaction of
3-crotonoyl-1,3-oxazolidin-2-one
to
cyclopentadiene
(eq 2),
13 and the ring opening of a meso anhydride to a half ester (eq 3).
18
19
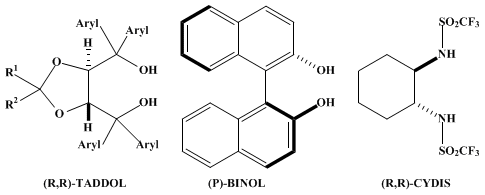
Note that the Ti-complexes of (R,R)-TADDOLs, (P)-BINOL, and the (R,R)-N,N'-1,2-cyclohexanediylbistrifluoromethanesulfonamide (CYDIS) usually give the same product enantiomer in a large variety of reactions, suggesting related transition states of the corresponding various reactions!
13
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(4R,5R)-2,2-Dimethyl-α,α,α',α'-tetra(naphth-2-yl)-1,3-dioxolane-4,5-dimethanol:
1,3-Dioxolane-4,5-dimethanol, 2,2-dimethyl-α,α,α',α'-tetra-2-naphthalenyl-, (4R-trans)-(12); (137365-09-4)
(R,R)-Dimethyl tartrate:
Tartaric acid, dimethyl ester, (+)- (8);
Butanedioic acid, 2,3-dihydroxy-, [R-(R,R)]-, dimethyl ester (9); (608-68-4)
2-Naphthylmagnesium bromide:
Magnesium, bromo-2-naphthyl- (8);
Magnesium, bromo-2-naphthalenyl- (9); (21473-01-8)
(R,R)-Dimethyl O,O-isopropylidenetartrate:
1,3-Dioxolane-4,5-dicarboxylic acid, 2,2-dimethyl-, dimethyl ester, (4R-trans)- (9); (37031-29-1)
Boron trifluoride etherate:
Ethyl ether, compd. with boron fluoride (1:1) (8);
Ethane, 1,1'-oxybis-, compd. with trifluoroborane (1:1) (9); (109-63-7)
Magnesium (8,9); (7439-95-4)
Iodine (8,9); (7553-56-2)
2-Bromonaphthalene:
Naphthalene, 2-bromo- (8,9); (580-13-2)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved