Org. Synth. 1999, 76, 169
DOI: 10.15227/orgsyn.076.0169
MONO-C-METHYLATION OF ARYLACETONITRILES AND METHYL ARYLACETATES BY DIMETHYL CARBONATE: A GENERAL METHOD FOR THE SYNTHESIS OF PURE 2-ARYLPROPIONIC ACIDS. 2-PHENYLPROPIONIC ACID
[
Benzeneacetic acid, α-methyl-
]
Submitted by Pietro Tundo, Maurizio Selva, and Andrea Bomben
1
.
Checked by Peter Belica, Robert Koehler, and Steven Wolff.
1. Procedure
Caution! The reaction must be carried out in a pressure vessel because it occurs at temperatures >160°C, and
dimethyl carbonate (DMC) boils at 90°C. An autoclave is recommended.
A stainless-steel (AISI 316) autoclave (internal volume 500 mL) equipped with a purging valve
(Note 1), a pressure gauge, a thermocouple, and a magnetic stir bar (Note 2), is charged with a mixture of
phenylacetonitrile (12.0 g, 0.10 mol),
dimethyl carbonate (DMC) (147.8 g, 1.64 mol), and
potassium carbonate (K2CO3) (28.4 g, 0.21 mol)
(Note 3), and is heated in an electrical oven at 180°C. The reaction mixture is magnetically stirred (900 rpm) for 18 hr (Note 4) in the autoclave at 180°C.
The autoclave is removed from the oven and cooled to room temperature. After the coproduct,
carbon dioxide
, is released by the purging valve, the autoclave is opened, and the pale-yellow suspension is transferred to a 500-mL separatory funnel
(Note 5). Water (120 mL) is added, and the mixture is extracted with
diethyl ether (3 × 60 mL). The combined organic extracts are dried over
sodium sulfate (25 g), which is then filtered off and washed with two
50-mL portions of diethyl ether
.
The solvent and excess
dimethyl carbonate are removed by rotary evaporation, and the remaining yellow liquid (
13.2 g of
2) is transferred to a
250-mL, round-bottomed flask equipped with a
condenser. An aqueous solution of
sodium hydroxide (10%, 60 mL) is added to the flask, and the mixture is heated with magnetic stirring in an
oil bath at reflux temperature for 4.5 hr
(Note 6). The course of the reaction is monitored by gas-chromatographic analysis
(Note 7). After the solution is cooled to room temperature, it is extracted with
diethyl ether to remove nonacidic material (primarily traces of amide). An aqueous solution of
hydrochloric acid (15%, 50 mL) is added portionwise
(Note 8). The suspension that forms is poured into a 250-mL separatory funnel and extracted with
diethyl ether (3 × 50 mL). The combined organic extracts are washed with water (60 mL) and dried over
sodium sulfate (25 g). After filtration, the solvent is removed by rotary evaporation, and the yellow liquid residue is distilled from a
25-mL flask, under reduced pressure, in a Claisen apparatus equipped with a
2-cm Vigreux column and a fused-on Liebig condenser. Distillation affords
2-phenylpropionic acid (3) (
14.3 g,
93%) as a pale-yellow liquid, bp
93-94°C/0.9 mm (lit.
2 bp
147°C/11 mm), with > 98% purity by gas chromatography
(Note 9).
2. Notes
1.
At room temperature, a stream of
nitrogen (about 400 mL/min for 3 min) is admitted through the purging valve to remove air before the reaction. The autoclave used by the checkers was charged, then pressurized with
nitrogen and vented three times before heating was initiated.
2.
The thermocouple is inserted into a
1/8-in stainless-steel pipe (fixed on the autoclave head) that dips into the reaction mixture. The
magnetic stir bar is 60 × 6 mm (length × diameter). The checkers used a
500-mL, electrically heated, stainless steel autoclave equipped with a
mechanical stirrer, a thermocouple, and a sampling tube.
3.
DMC is used in a large excess (15 molar excess with respect to the substrate) acting both as the methylating agent and the solvent. Previous investigations have been shown that
DMC as solvent provides a suitable polar-aprotic environment for the reaction and that it may be totally recovered (by distillation) after the reaction.
3
Potassium carbonate may also be used in catalytic amounts (5% molar with respect to the substrate), but longer reaction times result.
3
4.
Autogenic pressure reaches 12 bar (900 mm). Because of the presence of solid K
2CO
3, vigorous stirring is needed. Actually, the time required for complete substrate conversion is highly dependent on the rate of stirring of the mixture which, in turn, depends on the shape of the reaction vessel. Reaction times for the checkers' runs varied between 5 and 6.5 hr. If the reaction is not monitored by gas chromatography, a substantial amount of the undesired dimethylated product may form by further reaction of
2-phenylpropionitrile (2).
5.
A sample of a few drops of the mixture is diluted with
diethyl ether (2 mL), washed with water (2 mL), and analyzed by GC and GC/MS (DB5 capillary column, 30-m × 0.25-mm i. d., 0.25 μm film thickness). At a conversion of up to 99%, the yield of monomethyl derivative
2 (
2-phenylpropionitrile) is
98.5% while the dimethylated product is 0.15%.
6.
The temperature of the oil bath is 130°C. The initially observed biphasic (aqueous-organic) reaction system gradually turns to a homogeneous solution as the reaction proceeds.
7.
At intervals (1 hr), small aliquots (0.2-0.3 mL) of the reaction mixture are withdrawn, acidified with a few drops of concd
HCl (35%), and extracted with
diethyl ether (2 mL). Then they are analyzed by GC (DB5 capillary column); both the amide and the acid derived from the reacting nitrile are present. After 4.5 hr, the product is
99% acid
3 (
2-phenylpropionic acid). The checkers followed the hydrolysis by TLC analysis: SiO
2 plates; 4:1
hexane:ethyl acetate
; short-wave UV detection; R
f (acid) = 0.43, R
f (amide) = 0.61.
8.
Aliquots (5-7 mL) of the
HCl solution are added in 10 min. The final pH of the mixture is about 2.
9.
The product shows the following spectroscopic properties:
1H NMR (400 MHz, CDCl
3, TMS) δ: 1.51 (d, 3 H, J = 7.2, CH
3), 3.73 (q, 1 H, J = 7.2, CH), 7.31-7.33 (m, 4 H, Ph), 11.5-11.7 (br s, 1 H, OH)
;
13C NMR (100 MHz, CDCl
3, TMS) δ: 18.02, 45.36, 127.24, 127.56, 128.64, 139.68, 181.12
; mass spectrum (70 eV) m/z (relative intensity): 150 (M
+, 29), 106 (11), 105 (100), 104 (5),103 (12), 79 (14), 78 (6), 77 (17), 51 (7)
. Anal. Calcd for C
9H
10O
2: C, 71.98; H, 6.71. Found: C, 71.77; H, 6.69.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
2-Phenylpropionic acid is the simplest member of the 2-arylpropionic acids class to which a number of widely used antiinflammatory drugs belongs. Important examples of non-steroidal analgesics are 2-(p-isobutylphenyl)-, 2-(m-benzoylphenyl)- and 2-(6-methoxy-2-naphthyl)propionic acids well known commercially as Ibuprofen, Ketoprofen, and Naproxen, respectively.
Among the different synthetic procedures available for the preparation of hydratropic acids (e.g., indirect methylation of arylacetic acids,
4 asymmetric hydroformylation of styrenes,
5 rearrangements of α-bromoalkyl aryl ketals,
6 etc.), direct methylation of arylacetic acid derivatives seems the most attractive from both economical and synthetic aspects: the reagents are easily accessible and a one-pot reaction is involved. Nevertheless, this procedure is seldom used since the yields of the monomethyl derivatives are severely limited by the low selectivity of the reaction. Sizeable amounts of dimethylated by-products form.
3 Even under phase-transfer catalysis conditions, high selectivity in monomethylation is elusive.
7,8
The procedure described here overcomes this difficulty by using
dimethyl carbonate as a methylating agent.
9 In this case, although high reaction temperatures (> 180°C) are required, the methylation of CH
2-acidic compounds by DMC proceeds with a selectivity up to 99% toward the monomethyl derivatives, at complete conversion. The present reaction has been successfully carried out on a number of arylacetonitriles and methyl arylacetates
10 both on the multigram- (Table) and the gram-scale [2-(o-, m- and p-methoxyphenyl)-, 2-(o- and p-methylphenyl)-, 2-(p-fluorophenyl)-, 2-(p-chlorophenyl)-, 2-(m-carboxymethylphenyl)-propionitriles;
methyl 2-phenylpropionate].
3
TABLE
Monomethylation of Arylacetonitriles and Methyl Arylacetates with Dimethyl Carbonate.a
Hydrolysis of 2-Arylpropionitriles and Methyl 2-Arylopropionates to 2-Arylpropionic
|
|
Entry
|
Substrate
|
Reaction Time / hrc
|
Temp. (°C)c
|
Conv'n (%)c
|
Product
|
Yieldd (%)
|
|
|
1
|
|
18
|
180
|
>99
|
|
93
|
|
2
|
|
8.5
|
180
|
>99
|
|
94
|
|
3
|
|
9
|
180
|
>99
|
|
95
|
|
4
|
|
7.5
|
220
|
>98
|
|
84
|
|
aAll methylation reactions were carried out in a 500-ml autoclave using a substrate, DMC, and K2CO3 in a 1 : 16 : 2 molar ratio, respectively. Entries 1-3 and 4: 12.0 g and 8.0 g of substrate were used, respectively. bHydrolyses of the mono-methyl derivatives were carried out using a
10% aq solution of NaOH
(~ 5mL/g substrate) at reflux temperature. cReaction times, reaction temperature and conversions (determined by GC) refer to the methylation reaction. dYields refer ti distilled (bp: 93-94°C/0.9 mm, 116-118°C/0.3 mm, and 130-132°C/0.3 mm, entries 1-3, respectively) or recrystallized [
cyclohexane (60 mL/g); mp 150-151°C, entry 4] products.
|
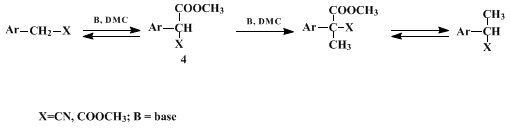
Mechanistic investigations of the reaction reported here suggest that the observed high selectivity may occur as a result of the double reactivity that DMC may exhibit.
3 It acts first as a methoxy carbonylating reagent (via a B
Ac2 mechanism) and then as a methylating agent (via a B
Al2 mechanism), as shown in Scheme 1. Selectivity arises from the fact that the reaction occurs only via the methoxy carbonylated intermediate (
4) and no direct methylation takes place on the ArCH
2X.
Beside the synthetic benefits of the procedure, the method also represents a true example of the
Green Chemistry concept intended as a new approach to synthesis, processing, and use of chemicals that reduces risks to the health and the environment.
11 In fact
DMC (now prepared by oxidative carbonylation of
methanol
12) is an innocuous methylating agent compared to the toxic methyl halides or
dimethyl sulfate
. Moreover, methylation processes by DMC are intrinsically environmentally benign in that neither organic by-products nor inorganic salts originate; conversely, alkylations by alkyl halides unavoidably lead to stoichiometric amounts of inorganic salts that must be disposed of.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Dimethyl carbonate:
Carbonic acid, dimethyl ester (8,9); (616-38-6)
Phenylacetonitrile: Aldrich:
Benzyl cyanide:
Acetonitrile, phenyl- (8);
Benzeneacetonitrile (9); (140-29-4)
2-Phenylpropionitrile:
Hydratroponitrile (8);
Benzeneacetonitrile, α-methyl- (9); (1823-91-2)
2-Phenylpropionic acid:
Hydratropic acid (8);
Benzeneacetic acid, α-methyl- (9); (492-37-5)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved