Org. Synth. 2000, 77, 186
DOI: 10.15227/orgsyn.077.0186
β-MERCAPTOPROPIONITRILE (2-CYANOETHANETHIOL)
[
Propanenitrile, 3-mercapto-
]
Submitted by R. Eric Gerber, Carlos Hasbun, Larisa G. Dubenko, Mei Fong King, and Donald E. Bierer
1
.
Checked by Joseph Barbosa and Amos B. Smith, III.
1. Procedure
Caution: The procedure should be carried out in a well-ventilated hood because of the extreme stench of the mercaptan products. All glassware used in the procedure should be soaked in a bleach solution prior to removal from the hood.
A.
2-Cyanoethylthiouronium hydrochloride
(3). To a 5-L, flanged-top, spherical Morton flask equipped with a supporting clamp
(Note 1) are added water (380 mL),
thiourea (575 g, 7.53 mol), and
3-chloropropionitrile (500 g, 5.58 mol)
(Note 2). The flask is equipped with a three-necked (with thermometer inlet) flanged-top, mechanical stirring rod (600 mm) with Teflon paddle (110 mm), temperature probe, reflux condenser, gas bubbler, and 5-L heating mantle. The reaction mixture is slowly heated to 68°C over a 30-min period under nitrogen and maintained at 68-70°C for 1 hr (Note 3). After 1 hr, the temperature of the reaction mixture is increased over a 15-min period to 100°C and maintained at 100-101°C for 2 hr (Note 4). After 2 hr, the heating mantle is replaced with a large ice-salt bath, and the reaction mixture is cooled with stirring to 45°C. When the internal temperature of the reaction mixture reaches 45°C, stirring is stopped, the cooling process is continued, and the product is allowed to crystallize (Note 5). Cold
acetone (2 L) is added and the solid is broken up with a spatula and homogenized (Note 6). The solid is collected using a 160 × 160-mm medium-fritted funnel and washed with
4 L of cold acetone
, stirring with the spatula during the filtration process. The solid is then washed with
2 L of ether
and air dried. Final drying in a vacuum oven at room temperature affords 693.7 g (75.0%) of the title compound as a white solid, mp 161.5-162.5°C
(Note 7) and (Note 8). The filtrate is placed in a freezer (4°C) for two days. The crystallized product is collected by vacuum filtration, washed with
ether (2 × 1 L), air dried, and then dried under vacuum to afford an additional 75.8 g (8.2%) of the title compound, mp 162.5-163.5°C
(Note 9).
B.
2-Cyanoethanethiol
(4). To a 3-L, three-necked, round-bottomed flask equipped with a supporting clamp are added
thiouronium salt 3 (369.8 g, 2.23 mol) and water (470 mL) (Note 10). The flask is equipped with a mechanical stirring rod (600 mm) with Teflon paddle (110 mm), temperature probe, gas bubbler, a Teflon tube that extends into the reaction mixture for bubbling nitrogen gas, and a 3-L heating mantle. The reaction mixture is purged with nitrogen by rapidly bubbling nitrogen into the reaction mixture with stirring for 15 min. The Teflon tube is removed, replaced with a 500-mL addition funnel, and then a concentrated solution of
sodium hydroxide (NaOH, 11.25 M, 4.24 mol) is slowly added under a nitrogen atmosphere, keeping the internal temperature below 25°C (Note 11). After the addition is complete, the reaction mixture is heated to 45°C over a 10-min period and held at 45-47°C for 45 min (Note 12). The heating mantle is removed and replaced with a large ice-salt bath. After the reaction mixture has cooled to 20°C, a 6 M solution of H2SO4 is slowly added under nitrogen, keeping the internal reaction temperature between 20-25°C, until the pH of the reaction mixture is 6 (Note 13). With rapid bubbling of nitrogen to maintain a nitrogen atmosphere, the addition funnel is removed and commercial anhydrous
ether (500 mL) is added to the reaction mixture. The flask is equipped with a one-hole rubber septum into which a Teflon tube (1 m × 4-mm id) is inserted. The mixture is stirred for 1 min, and the layers are allowed to separate. The temperature probe is removed and replaced with a rubber septum. Positive nitrogen pressure is applied to transfer the top ether layer using the Teflon tube into a 4-L Erlenmeyer flask with a 24/40 ground glass joint containing
magnesium sulfate (MgSO4, 375 g
) and a nitrogen atmosphere, cracking or removing the rubber septum on the thermometer inlet as needed to control the rate of transfer. This extraction process with ether is repeated four times using 500-mL portions. After the combined ether layers are dried over MgSO4 under nitrogen, they are filtered through a sintered glass funnel containing MgSO4 and concentrated using a rotary evaporator to about 500 mL in total volume. The concentrated ether solution is redried over MgSO4 (125 g, under nitrogen), filtered, and concentrated using the rotary evaporator. The product is transferred into a 250-mL, round-bottomed flask and distilled through a short-path jacketed distillation apparatus
(Note 14) and (Note 15). A forerun (approximately 5 mL) is collected and discarded. The product distilling at 30-32°C (0.08-0.12 mm) is collected, providing 106.6 g (55%) of the title compound as a colorless liquid (Note 16),(Note 17),(Note 18),(Note 19).
2. Notes
1.
The reaction can be carried out in a normal three-necked 5-L Morton flask. However, product
3 will solidify in the flask making its removal from a three-necked flask tedious. The submitter's experience has shown that use of an
open top reactor-type flask makes subsequent processing of the product much easier. The following glassware from Chemglass were used:
reactor flask, CG-1955-03;
clamp, CG-1970-01;
reaction vessel head, CG-1963-01;
air-free gas bubbler, AF-0514-02.
2.
Thiourea and 3-chloropropionitrile were purchased from Aldrich Chemical Company, Inc.
, and used as received.
Thiourea and 3-chloropropionitrile from Lancaster Laboratories
have also been used with similar results. Old bottles of
3-chloropropionitrile should be distilled before use.
3.
Care must be taken to heat the reaction mixture slowly and not above 70°C as the reaction mixture can exotherm above 100°C if heated rapidly to approximately 80°C. After the reaction mixture has reached 68°C, the reaction is self-perpetuating for about 30 min and the heating mantle should be removed. The heating mantle can be replaced periodically as necessary during the 1-hr heating time to maintain the temperature at 68-70°C when the reaction mixture begins to cool below 68°C. It is most convenient to use a soft, hemispherical 5-L GlasCol heating mantle that can be easily removed and replaced by slipping it around the flask without having to adjust the rest of the apparatus.
4.
The heating mantle can be removed and replaced (or turned off and on) as necessary during the 2-hr heating time to maintain the temperature at 100-101°C.
5.
If stirring is not stopped before the product solidifies, the stirring rod will break. It is best to raise the stirring rod and the temperature probe until they touch the top of the liquid in the flask. This assists the crystallization process and prevents supercooling.
6.
The
acetone used in the workup was precooled to −20°C for 4 hr prior to use. Once the solid is broken up, the
stirrer can be used to homogenize the product.
7.
The spectral data for
3 are as follows:
1H NMR (400 MHz, D
2O) δ: 2.83 (t, 2 H, J = 6.8), 3.28 (t, 2 H, J = 6.8)
;
13C NMR (100 MHz, D
2O) δ: 18.0, 26.2, 118.8, 169.5
; IR (KBr) cm
−1: 3275, 3095, 2710, 2241, 1651, 1441, 1411, 671
. The literature
2,3 mp for
3 is
163-165°C. Using
3-(trimethylsilyl)propanesulfonic acid, sodium salt as the internal reference for
1H NMR (δ = 0) and
methanol as the internal reference for
13C NMR (δ = 49.5), the checkers obtained the following NMR data:
1H NMR (500 MHz, D
2O) δ: 3.01 (t, 2 H, J = 6.7), 3.47 (t, 2 H, J = 6.7)
;
13C NMR (125 MHz, D
2O) δ: 18.6, 26.9, 119.4, 170.3
.
8.
Anal. Calcd for C
4H
8ClN
3S: C, 29.00; H, 4.87; N, 25.37; S, 19.36. Found: C, 28.75; H, 4.98; N, 25.51, S, 19.59.
9.
Anal. Calcd for C
4H
8ClN
3S: C, 29.00; H, 4.87; N, 25.37; S, 19.36. Found: C, 28.71; H, 4.93; N, 25.62; S, 19.68.
10.
The reaction mixture cooled to 12°C upon dissolution of
thiouronium salt
3. The checkers, however, found that complete dissolution of
3 was never achieved and that the internal temperature never fell below 15°C. A
water bath (ca. 15°C) was therefore used for added temperature control throughout the addition of the NaOH solution.
11.
The 11.25 M NaOH solution (prepared from
169.6 g of NaOH) should be cooled to 20°C prior to use.
12.
The temperature should not be allowed to go above 50°C. The heating mantle can be removed and replaced (or turned off and on) as necessary during the heating process to maintain the temperature at 45-47°C.
13.
Sulfuric acid (250 mL of 6 M H2SO4) was prepared and cooled to 20°C prior to use.
14.
Freshly opened cans of anhydrous
ether (Fisher) were used in the extraction process. Efficient drying over MgSO
4 (under
nitrogen) is needed to avoid excessive loss of product in the forerun as a product/water azeotrope. The one-hole rubber septum used for the transfer process was obtained by using a cork bore of the appropriate size on a 24/40 rubber septum (Aldrich Chemical Company, Inc.). Rotary evaporation to remove the ether was carried out at 200-300 mbar (150-225 mm) initially to prevent bumping. Final rotary evaporation to remove residual
ether prior to distillation was done at 40-50 mbar (30-38 mm).
15.
The use of a short path distillation apparatus with minimal joint connections, such as Chemglass CG-1240-06, is preferred. Once the distillation apparatus is assembled, vacuum should be gradually applied to remove any remaining residual ether before heat is applied. The receiving flask (500 mL) is placed in a Dewar flask containing a dry ice bath filled to a level to reach the bottom of the ground glass joint during the collection of
4. The
oil bath temperature during the distillation was kept between 50-60°C. Importantly, the vacuum pressure should be kept between 0.05 and 0.15 mm. Pressures in excess of 0.15 mm will lead to extensive thermal decompositon of product and pressures below 0.05 mm will result in loss of product due to volatility.
16.
The spectral data for
4 are as follows:
1H NMR (400 MHz, CDCl
3) δ: 1.78 (t, 1 H, J = 8.8), 2.62 (m, 2 H), 2.70-2.76 (m, 2 H)
;
13C NMR (100 MHz, CDCl
3) δ: 20.2, 22.4, 117.8
; IR (CCl
4) cm
−1: 2927, 2841, 2565, 2235, 1420, 1287
; MS (EI) 87 (M
+)
. The literature
3 bp for
4 is
57-59°C (6 mm).
17.
Analysis of
2-cyanoethanethiol by GCMS gave one peak at 87 amu with no detectable amount of dimer. The GCMS analysis was performed on a Hewlett-Packard 5890 Series II gas chromatograph equipped with a 5972 series mass selective detector and an
HP-5 30-m × 0.25-mm × 0.25-μm column under the following conditions: injector temp 150°C; detector temp 200°C; oven temp 50°C, 3 min; ramp 15°C/min; final temp 200°C;
helium gas flow 1.0 mL/min; t
R = 6.93 min. Anal. Calcd for C
3H
5NS: C, 41.35; H, 5.78; N, 16.07; S, 36.79. Found: C, 41.40; H, 5.85; N, 16.10; S, 37.03.
18.
3,3'-Dithiobispropionitrile
was prepared as a reference standard for GCMS analysis according to the procedure of Johnston and Gallagher and was recrystallized from ether.
2 Physical data for
3,3'-dithiobispropionitrile (unchecked) are as follows: mp
48.4-48.8°C (lit.
2
49-51°C);
1H NMR (400 MHz, CDCl
3) δ: 2.82 (m, 2 H), 2.94 (m, 2 H)
;
13C NMR (100 MHz, CDCl
3) δ: 17.8, 33.3, 117.7
; IR (KBr) cm
−1: 2937, 2239, 1411, 1322, 1266, 1204, 940, 895
; MS (EI) 172 (M
+)
. The GCMS analysis of
3,3'-dithiobispropionitrile using the above conditions gave one peak at t
R = 17.14 min (172 amu).
19.
The submitters routinely stored
2-cyanoethanethiol under
argon in a −78°C freezer prior to its use.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
2-Cyanoethylthiouronium hydrochloride
(3) has been prepared by Bauer and Welsh from
thiourea hydrochloride
and
acrylonitrile
in a
61% yield on a
20-g scale.
3 It has also been prepared by Bauer and Welsh
3 and by Traut, et al.
4 using a procedure similar to the one described here on a
17.5-g and
25-g scale, respectively. Both procedures describe the reaction being carried out at refluxing solvent temperatures. These procedures were not reproducible and applicable as described for larger scale preparations. More importantly, they were not safe, as the reaction of
thiourea with
2-chloropropionitrile is very exothermic. The procedure described here allows for the safe and reproducible preparation of
2-cyanoethylthiouronium hydrochloride on a scale even 10-fold larger than the one described.
2-Cyanoethanethiol
(4) has been prepared by Bauer and Welsh
3 and by Traut, et al.
4 using a procedure similar to the one described here. The Bauer and Welsh procedure employed less than a stoichiometric amount of NaOH while the Traut procedure used a small excess of NaOH; both were small scale preparations. The Traut procedure was not reproducible on small or larger scales, as yields never exceeded
35%. The submitters have found that increasing the amount of base during the hydrolysis reaction from that reported leads to an improved, reproducible yield of
2-cyanoethanethiol, provides product of high purity, and is applicable to large scale synthesis.
The 2-cyanoethyl moiety has demonstrated utility as a nitrogen
5 and sulfur protecting group.
6,7
2-Cyanoethanethiol has been used as a thiophile for the preparation of various 3-thio-substituted carbapenems.
8 More recently it has been used as a protected thiophile for the preparation of 1,2-dithiins, a novel heterocyclic class of compounds related to the biologically active natural products, thiarubrines A and B (
Figure 1).
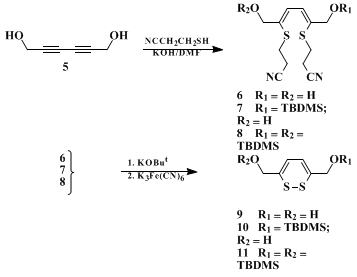
9,10 The 1,2-dithiin ring system can be constructed in two or three steps using
2-cyanoethanethiol as a key synthetic intermediate. Bis addition of
2-cyanoethanethiol to
2,4-hexadiyne-1,6-diol provides bisthiohexadiene adduct
6. Optional mono- or bis-silylation of the bisthiohexadiene adduct
6 provides hexadiene adducts
7 and
8, respectively. Removal of the 2-cyanoethyl moiety by β-elimination followed by oxidation of the intermediate dithioenolate provides dithiins
9,
10, and
11 (Scheme 1).
11 Desilylation of
dithiin
11 using
tetrabutylammonium fluoride provides an alternative approach to
dithiin
9. Dithiins
9 and
10 have been used to prepare a variety of 1,2-dithiin analogues, some of which are equipotent and less toxic than their natural product counterparts.
11,12
Figure 1

Thioacetate anion is a method of introducing
sulfur into organic molecules in a protected form.
13 The use of
2-cyanoethanethiol as the thiophile for the synthesis of 1,2-dithiins represents an important example where
thioacetate fails.
Benzylmercaptan
14,15 and
2-(trimethylsilyl)ethanethiol
16 have been used as protected thiophiles for the preparation of 1,2-dithiins by a similar mechanism. In the case of
benzylmercaptan, while the procedure works well for small scale preparations, process considerations hinder its use for larger applications.
11,16
2-(Trimethylsilyl)ethanethiol is commercially available and has been used successfully in the total synthesis of thiarubrine A,
16 yet its expense might limit its utility.
2-Cyanoethanethiol offers a practical alternative to both reagents. This preparation of
2-cyanoethanethiol should allow for its continued use as a thiophile and protected thiophile, for use of the 2-cyanoethyl moiety as a valuable protecting group for organic synthesis, and should also allow for further exploration of the 1,2-dithiin class of heterocycle.
17
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
β-Mercaptopropionitrile:
2-Cyanoethanethiol:
Propionitrile, 3-mercapto- (8);
Propanenitrile, 3-mercapto- (9); (1001-58-7)
2-Cyanoethylthiouronium hydrochloride:
Carbamimidothioic acid, 2-cyanoethyl ester, monohydrochloride salt (8,9); (6634-40-8)
Thiourea:
Urea, thio- (8);
Thiourea (9); (62-56-6)
3-Chloropropionitrile:
Propionitrile, 3-chloro- (8);
Propanenitrile, 3-chloro- (9); (542-76-7)
3-(Trimethysilyl)propanesulfonic acid, sodium salt:
1-Propanesulfonic acid,
3-(trimethylsilyl)-, sodium salt (8,9); (2039-96-5)
3,3'-Dithiobispropionitrile:
Propanenitrile, 3,3'-dithiobis- (9); (42841-31-6)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved