Org. Synth. 2002, 78, 104
DOI: 10.15227/orgsyn.078.0104
(PHENYL)[2-(TRIMETHYLSILYL)PHENYL]IODONIUM TRIFLATE. AN
EFFICIENT AND MILD BENZYNE PRECURSOR
[
Iodonium, phenyl-, 2-(trimethylsilyl)phenyl-, salt with trifluoromethane-sulfonic
acid
]
Submitted by Tsugio Kitamura
1a, Mitsuru Todaka
1b, and Yuzo Fujiwara
1c
.
Checked by Ralf Demuth and Rick L. Danheiser.
1. Procedure
A. 1,2-Bis(trimethylsilyl)benzene
(1). A dry, 500-mL, three-necked, round-bottomed flask is equipped
with a large Teflon-covered magnetic stir bar, 100-mL pressure-equalizing
addition funnel, Dimroth condenser
(Note 1)
fitted with a drying tube, and a glass stopper.
The flask is charged with
9.72 g
(0.400
mol) of magnesium turnings
(Note 2),
70 mL of hexamethylphosphoramide
(HMPA)
(Note 3),
11.25
mL
(0.100 mol) of 1,2-dichlorobenzene
(Note 4), and
0.254 g
(1.00 mmol) of iodine
(I2)
(Note 5). The addition funnel is charged with
51.0
mL
(0.400 mol) of freshly distilled chlorotrimethylsilane
(Note 6). The flask is immersed in an oil bath
at 70°C, stirring is initiated, and chlorotrimethylsilane is
added slowly, dropwise with vigorous stirring. After completion of the addition, the
oil bath is heated to 100°C and the reaction mixture is stirred at this temperature
for 2 days. During this time, the reaction mixture becomes viscous and finally separates
into two phases. The reaction mixture is cooled to ca. 40°C (Note 7)
and poured into a 1-L beaker containing saturated
sodium bicarbonate (NaHCO3) solution (200
mL),
diethyl ether
(100 mL), and ice (ca. 100 g). Solids and unreacted magnesium
metal are separated by suction filtration, the filtrate is transferred to a separatory
funnel, and the aqueous phase is extracted with three 150-mL portions
of ether. The combined ethereal extracts are washed with water (500 mL) and saturated sodium chloride (500 mL),
dried over anhydrous sodium sulfate
,
and filtered. The solvent is evaporated under reduced pressure and the residue is
distilled from a 100-mL, round-bottomed flask with a magnetic stir bar
through a 20-cm Vigreux column at reduced pressure. The fraction boiling at 128-133°C
(20 mm) is collected to afford 16.5-16.7
g (74-75%) of
1,2-bis(trimethylsilyl)benzene
(1) (Note 8)
as a colorless liquid.
B. (Phenyl)[2-(trimethylsilyl)phenyl]iodonium triflate
(2). A 100-mL round-bottomed flask equipped with an argon inlet
adapter and a magnetic stir bar is charged with
12.9
g
(40.0 mmol) of finely ground (diacetoxyiodo)benzene
(Note 9) and
70 mL
of dichloromethane
(Note 10).
The suspension is cooled at 0°C with an ice bath and
6.9 mL
(78 mmol) of trifluoromethanesulfonic
acid
(Note 11) is added in one portion
by syringe. The resulting clear yellow solution is stirred at room temperature for
2 hr and a solution of
8.9 mL
(40.0
mmol) of 1,2-bis(trimethylsilyl)benzene
in
10 mL of dichloromethane
is added dropwise by syringe. The resulting mixture is stirred at room temperature
for 12 hr, and the solvent is removed by rotary evaporation under reduced pressure
to afford the product as colorless crystals. (When an oily residue is obtained, it
can be crystallized by triturating with diethyl ether.) The crystals
are collected by filtration and washed with
40 mL
of diethyl ether
to afford 14.7-15.7 g (73-78%)
of
(phenyl)[2-(trimethylsilyl)phenyl]iodonium
triflate
(2) as colorless needles, mp 142-143°C
(Notes 12, 13).
C. Generation of benzyne and trapping with furan.
A 50-mL round-bottomed flask fitted with a pressure-equalizing
addition funnel equipped with an argon inlet adapter
and a magnetic stir bar is charged with
1.51
g
(3.00 mmol) of (phenyl)[2-(trimethylsilyl)phenyl]iodonium
triflate
(2),
10 mL
of dichloromethane
, and
1.10
mL
(15.1 mmol) of furan
(Note 14). The addition funnel is charged with
3.6
mL
(3.6 mmol) of 1.0 M tetrabutylammonium fluoride
(Bu4N+F−) in
tetrahydrofuran
(THF)
(Note 15). The flask is placed in an ice
bath and the tetrabutylammonium fluoride solution
is added dropwise over ca. 5 min. The reaction mixture is stirred at room temperature
for 30 min, and water (20 mL) is added. The aqueous phase is separated and extracted
with three
10-mL portions of dichloromethane
.
The combined organic extracts are washed with 15 mL of water, dried over anhydrous sodium sulfate
, and filtered.
The solvent is evaporated by rotary evaporation under reduced pressure, and the residual
oil is purified by column chromatography through
60
g of silica gel packed in a 4-cm diameter column (elution with dichloromethane)
to give 0.415-0.418 g (96-97%) of
1,4-dihydronaphthalene
1,4-oxide
(3) as colorless crystals, mp 52-55°C (Notes 16, 17).
2. Notes
1.
A highly efficient
reflux condenser is
required to avoid the loss of
chlorotrimethylsilane by evaporation
during the reaction.
2.
Magnesium turnings
were purchased from Nacalai Tesque, Inc. or Fisher Scientific Company
.
3.
HMPA,
hexamethylphosphoramide
, is toxic and a
cancer-suspect agent. It was purchased from Tokyo Kasei Kogyo Co. or Aldrich Chemical
Company, Inc. and distilled from
calcium hydride under reduced
pressure before use. When
dimethylpropyleneurea (DMPU) was used
in place of HMPA, none of the desired
bis(trimethylsilyl)benzene
was obtained.
4.
1,2-Dichlorobenzene
was purchased by the submitters from Tokyo Kasei Kogyo Co.
and distilled under reduced pressure. The checkers used
99%
anhydrous 1,2-dichlorobenzene from Aldrich Chemical
Company, Inc.
, without further purification.
5.
Iodine was purchased
from Tokyo Kasei Kogyo Co. or Aldrich Chemical Company,
Inc.
, and used as received.
6.
The submitters purchased
chlorotrimethylsilane
from Shin-Etsu Chemicals
and distilled it prior to
use. The checkers used
99+%
chlorotrimethylsilane
from Aldrich Chemical Company, Inc.
, without further
purification.
7.
If cooled to room temperature, the lower layer solidifies.
8.
Bis(trimethylsilyl)benzene (
1) has
the following spectral properties:
1H
NMR (300 MHz, CDCl
3) δ: 0.36 (s, 18 H), 7.28-7.34
(m, 2 H), 7.64-7.68 (m, 2 H)
;
13C NMR (75 MHz, CDCl
3) δ:
2.0, 127.8, 135.2, 146.0
.
9.
(Diacetoxyiodo)benzene
was purchased from Aldrich Chemical Company, Inc.
,
and was used as received.
10.
Dichloromethane was distilled from
phosphorus pentoxide (P2O5)
or
calcium hydride
prior
to use.
11.
Trifluoromethanesulfonic
acid from Central Glass Co. or Aldrich Chemical
Company, Inc.
, was employed.
12.
Product
2 has the following spectral properties:
1H NMR (400 MHz, CDCl
3)
δ: 0.42 (s, 9 H), 7.26-8.13 (m, 9 H)
;
13C NMR (100 MHz, CDCl
3)
δ: 0.1, 114.0, 121.2, 132.2,
133.2, 133.4, 138.5, 139.1, 147.3
.
13.
The submitters obtained
2 in
86% yield.
14.
Furan was purchased
from Tokyo Kasei Kogyo Co. or Aldrich Chemical Company,
Inc.
, and distilled prior to use.
15.
1.0 M Tetrabutylammonium
fluoride in THF was obtained from Aldrich Chemical Company, Inc.
16.
If the product is obtained as an oil, it is cooled in a −78°C
bath to induce crystallization.
17.
Product
3 has the following spectral properties:
1H NMR (400 MHz, CDCl
3)
δ: 5.69 (s, 2 H), 6.94-6.96 (m, 2 H), 7.00 (s,
2 H), 7.22-7.24 (m, 2 H)
;
13C NMR (100 MHz, CDCl
3) δ:
82.2, 120.2, 124.9, 142.9, 148.9
.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Benzyne is one of a group of reactive intermediates widely applicable to organic
synthesis.
2
3
4
5 The title hypervalent
iodine-benzyne
precursor,
(phenyl)[2-(trimethylsilyl)phenyl]iodonium triflate
2,
6 is prepared by only
two steps from commercially available reagents. Products
1 and
2 are
stable and easily purified. The hypervalent
iodine-benzyne precursor
2 is obtained as a stable solid and handled without any precautions. More importantly,
benzyne is generated by using
tetrabutylammonium fluoride under
mild and neutral conditions. Therefore, compound
2 is useful for reactions
of substrates that cannot be conducted at high temperatures or under basic conditions.
The advantages of the use of this hypervalent iodine-benzyne
precursor 2 are as follows: (1) The benzyne precursor
2 is a stable crystalline compound up to its
melting point, usually to 130°C.
(2) The benzyne precursor 2 is not hygroscopic and is stable to air; it can
be handled without any special precautions. (3) The generation of benzyne can be conducted
under neutral conditions and at room temperature.
The high efficiency of the present precursor
2 is demonstrated by comparison
with a similar precursor,
2-(trimethylsilyl)phenyl triflate (
4),
which generates benzyne under mild conditions (room temperature and neutral).
7 Benzyne precursor
2 gives the
adduct,
1,4-epoxy-1,4-dihydronaphthalene
3, quantitatively
in the reaction with
furan, while the reaction of benzyne precursor
4 under the same conditions leads to a lower yield of adduct
3 and needs
longer reaction time.
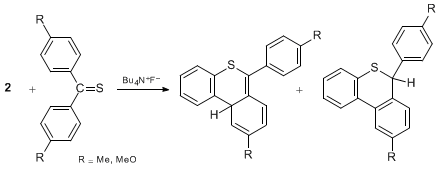
The reaction of thiobenzophenones with benzyne shows the superiority of the present
iodine precursor
2 over
benzenediazonium-2-carboxylate
(
5), which is widely used.
8 The reaction of the hypervalent
iodine
precursor
2 with thiobenzophenones affords [4+2] cycloadducts from benzyne
and thiophenzophenones under mild conditions. However, the reaction with
benzenediazonium-2-carboxylate
5 gives no benzyne adducts, but benzoxathianones, which are presumably derived
from the reaction of 2-carboxyphenyl cation and cyclization.
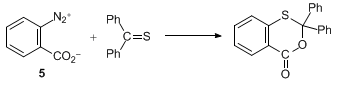
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
(Phenyl) [2-(trimethylsilyl)phenyl]iodonium triflate:
Iodonium, phenyl-, 2-(trimethylsilyl)phenyl-, salt with trifluoromethanesulfonic
acid (1:1) (13); (164594-13-2)
1,2-Bis(trimethylsilyl)benzene:
Silane, o-phenylenebis[trimethyl-
(8);
Silane, 1,2-phenylenebis[trimethyl- (9); (17151-09-6)
Magnesium (8,9); (7439-95-4)
Hexamethylphosphoramide: HIGHLY TOXIC: CANCER SUSPECT
AGENT:
Phosphoric triamide, hexamethyl- (8,9) (680-31-9)
l,2-Dichlorobenzene;
Benzene, o-dichloro-
(8);
Benzene, 1,2-dichloro- (9); (95-50-1)
Iodine (8,9); (7553-56-2)
Chlorotrimethylsilane:
Silane, chlorotrimethyl-
(8,9); (75-77-4)
(Diacetoxyiodo)benzene: Aldrich:
Iodobenzene
diacetate:
Benzene, (diacetoxyiodo)- (8);
Iodine,
bis(aceto-O)phenyl- (9); (3240-34-4)
Trifluoromethanesulfonic acid: HIGHLY CORROSIVE:
Methanesulfonic
acid, trifluoro- (8,9); (1493-13-6)
Furan (8,9); (110-00-9)
Tetrabutylammonium fluoride:
Ammonium, tetrabutyl-,
fluoride (8);
1-Butanaminium, N,N,N-tributyl-, fluoride
(9); (429-41-4)
1,4-Dihydronaphthalene 1,4-oxide:
1,4-Epoxy-1,4-dihydronaphthalene:
1,4-Epoxynaphthalene, 1,4-dihydro- (8,9); (573-57-9)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved