Org. Synth. 2002, 78, 202
DOI: 10.15227/orgsyn.078.0202
PREPARATION AND DIELS-ALDER REACTION OF A 2-AMIDO SUBSTITUTED FURAN: tert-BUTYL
3a-METHYL-5-OXO-2,3,3a,4,5,6-HEXAHYDROINDOLE-1-CARBOXYLATE
[
1H-Indole-1-carboxylic acid, 2,3,3a,4,5,6-hexahydro-3a-methyl-5-oxo-,
1,1-dimethylethyl ester
]
Submitted by Albert Padwa
1
, Michael A. Brodney, and Stephen M. Lynch.
Checked by Sivaraman Dandapani and Dennis P. Curran.
1. Procedure
A. Furan-2-ylcarbamic acid tert-butyl ester
.
In a 250-mL, one-necked, round-bottomed flask equipped with
a magnetic stirring bar are placed
10
g (0.07 mol) of 2-furoyl chloride
(Note 1),
80 mL of
tert-butyl alcohol
(Note 2),
and
5.1 g (0.08 mol) of sodium
azide
(Note 3). After the flask is stirred
at 25°C for 20 hr under an argon atmosphere, it is placed behind
a protective shield (Note 4) and the solution is heated at reflux
for 15 hr under a constant flow of argon. The solvent is removed
with a rotary evaporator at aspirator vacuum to provide a white
solid that is purified by flash silica gel chromatography (
10%
ethyl acetate/hexane
) to give
10.8 g (81%) of
furan-2-ylcarbamic
acid tert-butyl ester
as a white
solid: mp 98-99°C
(Note 5).
B. 4-Bromo-2-methyl-1-butene. In a
500-mL, three-necked, round-bottomed flask equipped with a
magnetic stirring bar, reflux condenser,
and a dropping funnel are placed
20
g (0.23 mol) of 3-methyl-3-buten-1-ol
(Note 6),
160 mL of
freshly distilled dichloromethane
(Note 7)
and
36 mL (0.24 mol) of triethylamine
(Note 8). The reaction mixture is cooled to −5°C and
14.4 g (0.24 mol) of freshly distilled
methanesulfonyl chloride
(Note 9)
is added dropwise from the dropping funnel. After the reaction mixture is stirred
at 0°C for an additional 2 hr, it is quenched with 80 mL of water and the aqueous
phase is extracted three times with
40-mL
portions of dichloromethane
. The combined organic
phase is dried over
magnesium sulfate
and the solvent is removed with a rotary evaporator at aspirator vacuum. The crude
yellow oil is taken up in
25 mL of dry acetone
and added dropwise from a dropping funnel to a slurry of
60
g (0.68 mol) of lithium bromide
in
115 mL of dry acetone
in a 250-mL round-bottomed flask fitted with the dropping
funnel and a reflux condenser. The solution is
slowly warmed to 35°C and is stirred at this temperature for 18 hr, at which time
the reaction is quenched with 120 mL of water. The aqueous phase is extracted three
times with
40-mL portions of ether.
The combined organic phase is dried over
magnesium
sulfate
and the solvent is removed with a rotary evaporator
at aspirator vacuum. The resulting oil is distilled at aspirator pressure to provide
17.7 g (51%) of
4-bromo-2-methyl-1-butene
as a colorless oil: bp 41-42°C at 34-39 torr
(Note 10).
C. tert-Butyl N-(3-methyl-3-butenyl)-N-(2-furyl)carbamate.
In a flame-dried, 500-mL, one-necked, round-bottomed flask
equipped with a magnetic stirring bar and reflux
condenser are placed
4.0 g (21.8
mmol) of furan-2-ylcarbamic acid tert-butyl ester
and
150 mL of toluene
(Note 11) under an argon atmosphere. To
this solution are added
3.1 g (76.4 mmol)
of freshly ground powdered sodium hydroxide
,
6.04 g (43.7 mmol) of potassium
carbonate
, and
1.48 g
(4.4 mmol) of tetrabutylammonium hydrogen sulfate
(Note 12). The solution is heated at 80°C for 25 min with vigorous
stirring and then
3.9 g (26.2 mmol)
of freshly distilled 4-bromo-2-methyl-1-butene
is
added as a solution in
10 mL of toluene
over a 30-min period. After being heated at 80°C for 30 min, the solution is charged
with an additional
0.98 g (6.6 mmol)
of 4-bromo-2-methyl-1-butene
. The mixture is heated
at 80°C for an additional 1 hr. After the reaction is cooled to room temperature,
it is quenched by the addition of 200 mL of water and the aqueous phase is extracted
three times with
100-mL portions of dichloromethane
.
The combined organic phase is dried over
magnesium
sulfate
and the solvent is removed with a rotary evaporator
at aspirator vacuum. The crude residue is purified by silica gel chromatography (
10%
ethyl acetate-hexane
)
to give 5.0 g (91%) of
tert-butyl
N-(3-methyl-3-butenyl)-N-(2-furyl)carbamate
as a colorless oil (Note 13).
D. tert-Butyl 3a-methyl-5-oxo-2,3,3a,4,5,6-hexahydroindole-1-carboxylate.
Into an oven-dried, 35-mL heavy-wall, high pressure tube
(Note 14) equipped with a magnetic stirring bar
and rubber septum are placed
3.7
g (14.7 mmol) of tert-butyl N-(3-methyl-3-butenyl)-N-(2-furyl)carbamate
and
20 mL of toluene
under an argon atmosphere. Argon is vigorously
bubbled through the solution for 30 min at which time the septum is replaced with
a threaded plunger valve equipped with an O-ring seal
(Note 14). The vessel is placed behind a protective shield
(Note 4) and immersed into a
preheated oil bath at 160°C for 14 hr. After the solution is
cooled to room temperature, solvent is removed with a rotary evaporator at aspirator
vacuum and the crude residue is purified by silica gel chromatography (
40%
ethyl acetate-hexane
) to give
2.8 g (70-75%) of
tert-butyl
3a-methyl-5-oxo-2,3,3a,4,5,6-hexahydroindole-1-carboxylate
as
a white solid: mp 112-113°C
(Note 15).
2. Notes
1.
2-Furoyl chloride
was purchased from Aldrich Chemical Company, Inc.
,
and used without further purification.
2.
2-Methyl-2-propanol
(HPLC grade; tert-butyl alcohol) was purchased from Aldrich
Chemical Company, Inc.
, and used without further purification.
3.
Sodium azide (99%)
was purchased from Aldrich Chemical Company, Inc.
; a
Teflon spatula was
used when handling this reagent.
Caution: avoid contact with metal
and heat when using sodium azide
.
4.
The protective shield was purchased from Lab-Line, Inc. and was used for
protection when heating at high temperatures.
5.
The product has the following spectralcharacteristics: IR (neat) cm
−1: 3267,
2980, 1700, and 1546
;
1H NMR (CDCl
3,
300 MHz) δ: 1.50 (s, 9 H), 6.04 (brs, 1 H), 6.34
(m, 1 H), 6.63 (brs, 1 H), and 7.06 (m, 1 H)
;
13C NMR (CDCl
3,
75 MHz) δ: 28.2, 81.3, 95.1, 111.2,
136.0, 145.4, 151.9
. Anal. Calcd
for C
9H
13NO
3: C, 59.00; H, 7.15; N, 7.64. Found:
C, 59.09; H, 7.13; N, 7.67.
6.
3-Methyl-3-buten-1-ol
was purchased from Aldrich Chemical Company, Inc.
,
and used without further purification.
7.
Dichloromethane was distilled from
calcium
hydride prior to use.
8.
Triethylamine was
purchased from Aldrich Chemical Company, Inc.
, and
used without further purification.
9.
Methanesulfonyl chloride
was purchased from Aldrich Chemical Company, Inc.
,
and was distilled before use.
10.
The product has the following spectral characteristics: IR (neat) cm
−1: 3075,
1652, 1445, and 890
;
1H NMR (CDCl
3, 400 MHz) δ:
1.75 (s, 3 H), 2.58 (t, 2 H, J = 7.4), 3.47 (t, 2
H, J = 7.4), 4.77 (s, 1 H), and 4.86 (s, 1 H)
;
13C NMR (CDCl
3,
100 MHz) δ: 22.1, 31.0, 41.1, 112.9,
and 142.6
11.
Toluene was distilled from
calcium
hydride prior to use.
12.
Tetrabutylammonium hydrogen
sulfate (97%) was purchased from Aldrich Chemical
Company, Inc.
, and used without further purification.
13.
The product has the following spectral characteristics: IR (neat) cm
−1: 2975,
1711, 1606, and 1369
;
1H NMR (CDCl
3,
400 MHz) δ: 1.45 (s, 9 H), 1.74 (s, 3 H), 2.27
(t, 2 H, J = 7.2), 3.67 (dd, 2 H, J = 9.2 and 6.0), 4.71
(s, 1 H), 4.76 (s, 1 H), 6.33 (brs, 1 H), 7.14
(t, 1 H, J = 1.2), and 6.0 (brs, 1 H)
;
13C NMR (CDCl
3, 100 MHz) δ:
22.2, 28.0, 36.6, 46.9, 80.7,
100.9, 110.7, 111.8, 137.8, 142.3,
148.3, and 153.5
. Anal. Calcd for C
14H
21NO
3:
C, 66.91; H, 8.42; N, 5.57. Found: C, 66.93; H, 8.38; N, 5.60. The broad resonance
at δ 6.0 in the
1H NMR spectrum merges into a sharp multiplet when
the spectrum is recorded at 50°C.
14.
The 35-mL heavy-wall high pressure tube, Teflon plug, and O-ring
were purchased from Ace Glass and were oven dried prior to use.
15.
The product has the following spectral characteristics: IR (KBr) cm
−1: 2961, 1709,
and 1388
;
1H
NMR (DMSO-d
6, 400 MHz) δ: 0.94 (s, 3 H), 1.42
(s, 9 H), 1.72 (m, 2 H), 2.35 (d, 1 H, J = 14.6),
2.49 (d, 1 H, J = 14.6), 2.63 (dd, 1 H, J = 14.6 and 2.8),
2.89 (dd, 1 H, J = 14.6 and 4.8), 3.51 (m, 1 H), 3.66
(m, 1 H), and 5.78 (brs, 1 H)
;
13C NMR (DMSO-d
6, 100 MHz) δ:
22.8, 27.7, 35.2, 36.7, 42.5,
46.1, 51.6, 79.6, 96.4, 143.7,
151.5, and 208.3
. Anal. Calcd for C
14H
21NO
3:
C, 66.91; H, 8.42; N, 5.57. Found: C, 66.99; H, 8.38; N, 5.49.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Heterocycles such as
furan,
thiophene,
and
pyrrole undergo Diels-Alder reactions despite their stabilized
6p-aromatic electronic configuration.
2 By far the
most extensively studied five-ring heteroaromatic system for Diels-Alder cycloaddition
is furan and its substituted derivatives.
3
The resultant 7-oxabicyco[2.2.1]heptanes are valuable synthetic intermediates that
have been further elaborated to substituted arenes, carbohydrate derivatives, and
various natural products.
4
5
6 A crucial
synthetic transformation employing these intermediates involves the cleavage of the
oxygen bridge to produce functionalized cyclohexene derivatives.
7,8
While the bimolecular Diels-Alder reaction of alkyl-substituted furans has been the
subject of many reports in the literature,
9
much less is known regarding the cycloaddition behavior of furans that contain heteroatoms
attached directly to the aromatic ring.
10 In this regard, we have become
interested in the Diels-Alder reaction of 2-aminofurans as a method for preparing substituted
aniline derivatives since these compounds are important starting materials for the
preparation of various pharmaceuticals.
11 Many furan Diels-Alder reactions
require high pressure or Lewis acid catalysts to give satisfactory yields of cycloadduct.
12
In contrast to this situation,
2-amino-5-carbomethoxyfuran readily
reacted with several monoactivated olefins by simply heating in
benzene
at 80°C. The initially formed cyclohexadienol underwent a subsequent dehydration when
treated with 1 equiv of
boron trifluoride etherate (BF
3·OEt
2)
to give the substituted aniline derivative.
13 In each case, the cycloaddition proceeded with complete
regioselectivity, with the electron-withdrawing group being located ortho to the amino
group. The regiochemical results are perfectly consistent with FMO theory.
14 The most favorable FMO
interaction is between the HOMO of the furanamine and the LUMO of the dienophile.
The atomic coefficient at the ester carbon of the furan is larger than at the amino
center, and this nicely accommodates the observed regioselectivity.
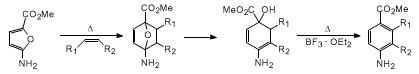
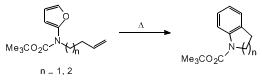
The intramolecular Diels-Alder reaction of furans, often designated as IMDAF,
15 helps
to overcome the sluggishness of this heteroaromatic ring system toward [4+2]-cycloaddition.
Not only do IMDAF reactions allow for the preparation of complex oxygenated polycyclic
compounds, but they also often proceed at lower temperatures than their intermolecular
counterparts.
9 Even more significantly, unactivated
p-bonds are often suitable dienophiles for the internal cycloaddition. Indeed, the
submitters discovered that the IMDAF reaction of a series of furanamide derivatives
occurred smoothly to furnish cyclized aromatic carbamates as the only isolable products
in high yield.
16
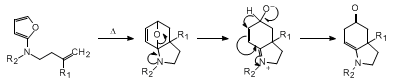
When the alkenyl group possesses a substituent at the 2-position of the p-bond, the
thermal reaction furnishes a rearranged hexahydroindolinone.
17
With this system, the initially formed cycloadduct cannot aromatize. Instead, ring
opening of the oxabicyclic intermediate occurs to generate a zwitterion that undergoes
hydride transfer to give the rearranged ketone. The procedure described here provides
a simple and general approach for the construction of various hexahydroindolinones.
This strategy can be cleanly applied toward the synthesis of more complex octahydroindole-based
alkaloids.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
tert-Butyl 3a-methyl-5-oxo-2,3,3a,4,5,6-hexahydroindole-1-carboxylate:
1H-Indole-1-carboxylic acid, 2,3,3a,4,5,6-hexahydro-3a-methyl-5-oxo-, 1,1-dimethylethyl
ester (14); (212560-98-0)
Furan-2-ylcarbamic acid tert-butyl ester:
Carbamic
acid, 2-furanyl-, 1,1-dimethylethyl ester (9); (56267-47-1)
2-Furoyl chloride (8):
2-Furancarbonyl chloride
(9); (527-69-5)
tert-Butyl alcohol (8):
2-Propanol, 2-methyl-
(9); (75-65-0)
Sodium azide (8,9); (26628-22-8)
4-Bromo-2-methyl-1-butene:
1-Butene, 4-bromo-2-methyl-
(8,9); (20038-12-4)
3-Methyl-3-buten-1-ol:
3-Buten-1-ol, 3-methyl-
(8,9); (763-32-6)
Methanesulfonyl chloride (8,9); (124-63-0)
Lithium bromide (8,9); (7550-35-8)
tert-Butyl N-(3-methyl-3-butenyl)-N-(2-furyl)carbamate:
Carbamic acid, 2-furanyl(3-methyl-3-butenyl)-, 1,1-dimethylethyl ester
(14); (212560-95-7)
Toluene (8);
Benzene, methyl-
(9); (108-88-3)
Tetrabutylammonium hydrogen sulfate:
Ammonium,
tetrabutyl-, sulfate (1:1) (8);
1-Butanaminium, N,N,N-tributyl-,
sulfate (1:1) (9); (32503-27-8)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved