Checked by Lloyd J. Simons and William R. Roush.
1. Procedure
2. Notes
1.
(−)- and (+)-Norephedrine,
benzyl chloride, tetrabutylammonium iodide,
and propionyl chloride were purchased from Wako Pure
Chemical Ltd. Japan or Aldrich Chemical Company, Inc.
,
and used as received.
mesitylenesulfonyl chloride
was obtained from Tokyo Kasei Kogyo Ltd. Japan or Aldrich
Chemical Company, Inc.
and used as received.
Dichloromethane
and
acetonitrile
were distilled
from
calcium hydride (CaH2)
under an inert atmosphere prior to use.
Merck
60 silica gel, 0.040-0.063 mm, or
Whatman 60 Å
230-400 mesh silica gel was used for column chromatography.
2.
The submitter reports that a second crop of pure
2 (
6.0 g) could be obtained by concentration
and recrystallization of the mother liquors. However, the second crops obtaine by
the checkers were highly colored and were not sufficiently pure for use in the next
step.
3.
Sulfonamide
2
exhibited the following physical and spectroscopic properties:
mp 121-122°C, TLC (silica gel) R
f = 0.28 in 3:1 hexanes
:
ethyl acetate; (1R, 2S)-
2:
[α]D
23 −12.4 (c 2.12, CHCl3).
(
1S, 2R)-
2:
[α]D
23
+12.8 (c 2.12, CHCl3).
1H NMR (500 MHz, CDCl
3) (concentration dependent;
20 mg/mL) δ: 0.87 (d, 3 H, J = 6.8), 2.30 (s, 3 H),
2.52 (1 H, -OH), 2.66 (s, 6 H), 3.53 (m, 1
H), 4.76 (br,1 H, -NH), 4.82 (d,1 H, J = 8.8),
6.96 (s, 2 H), 7.20-7.36 (m, 5 H)
; (200 mg/mL) δ: 0.88 (d, 3 H, J = 6.8),
2.32 (s, 3 H), 2.68 ( s, 6 H), 3.08 (1 H, -OH),
3.51 (ddq, 1 H, J = 3.1, 6.8, 9.0), 4.81 (br, 1 H, -NH),
5.23 ( br, 1 H), 6.98 (s, 2 H), 7.20-7.36 (m, 5 H)
.
13C NMR (125
MHz, CDCl
3, c = 20 mg/mL) δ: 14.3, 20.9,
22.9, 54.6, 75.6, 125.9, 127.5,
128.3, 132.0, 134.2, 138.9, 140.5,
142.2
. IR (thin film
from CH
2Cl
2) cm
−1: 3504, 3302,
2981, 1064, 1452, 1320, 1156,
1058, 972, 895, 702, 657
.
HRMS-FAB: Calcd for C
18H
24NO
3S [M+H]+,
334.1477
m/z
; Found,
334.1478
m/z
.
Anal. Calcd for C
18H
23NO
3S: C, 64.84; H, 6.95; N,
4.20. Found: C, 64.94; H, 6.98; N, 4.17.
4.
The checkers observed that the reaction is not complete according
to TLC analysis after the prescribed 17-hr reaction period. However, if the reaction
is allowed to proceed for longer reaction times, the dibenzylated product
4
is obtained with correspondingly diminished yields of
3. When the reaction
was terminated after 17 hr, the checkers obtained an
86%
yield of recrystallized
3.
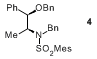
5.
The submitter reports that an additional
3.2 g (
15%)
of
3 was isolated by chromatography
(Note 1) of the mother
liquor on silica gel (100 g) with
10%
ethyl acetate
in
hexane
.
The checkers observed that the concentrated mother liquors were not soluble in the
chromatography solvent, so they dissolved the viscous oil in
dichloromethane
(<7 mL) and applied this solution to the column. Once
this material was adsorbed, the column was flushed with
hexanes
(400 mL) to remove the CH
2Cl
2. Elution
of the column with
10%
ethyl
acetate-hexanes then provided additional product
2. The
combined yield of
2 obtained by the checkers via the crystallization and chromatography
sequence was
90-93%.
6.
N-Benzylsulfonamide
3
exhibited the following physical and spectroscopic properties:
mp 123-124°C; TLC (silica gel) R
f = 0.48 in
3:1 hexanes :
ethyl acetate
;
R
f = 0.15 in 9:1 hexanes :
ethyl acetate; (
1R,
2S)-
3:
[α]D
23
−6.3° (c 2.06, CHCl3); (
1S, 2R)-
3:
[α]D
23 +6.4° (c 2.05, CHCl3);
1H NMR (500 MHz,
CDCl
3) (concentration dependent; 18 mg/mL) δ: 1.03 (d, 3 H,
J = 7.0), 2.14 (1 H, -OH), 2.29 (s, 3 H),
2.65 (s, 6 H), 3.82 (dq, 1 H, J = 1.9, 7.0), 4.54
(1 H, A of ABq, J
AB = 16.1), 4.77 (1 H, B of ABq, J
AB
= 16.1), 5.00 (br s, 1 H), 6.93 (s, 2 H), 7.04-7.08
(m, 2 H), 7.10-7.36 (m, 8 H)
; (218 mg/mL) δ: 1.02 (d, 3 H, J = 7.0 ), 2.27
(s, 3 H), 2.34 (1 H, -OH), 2.62 (s, 6 H),
3.82 (dq, 1 H, J = 1.9, 7.0), 4.56 (1 H, A of ABq, J
AB
= 16.1), 4.75 (1 H, B of ABq, J
AB = 16.1 Hz), 4.96
(br s, 1 H), 6.91 (s, 2 H), 7.04-7.08 (m, 2 H), 7.10-7.36
(m, 8 H)
;
13C
NMR (125 MHz, CDCl
3, 18 mg/mL) δ: 9.8, 20.9,
23.0, 49.1, 59.7, 76.6, 125.5,
127.2, 127.4, 127.7, 128.2, 128.6,
132.2, 133.4, 138.6, 140.2, 142.1,
142.6
; IR (film from
CH
2Cl
2) cm
−1: 3502, 2981,
1604, 1454, 1314, 1150, 1022,
924, 855, 699, 658
;
HRMS-FAB: Calcd for C
25H
30NO
3S [M+H]
+,
424.1946
m/z
;
Found,
424.1947
m/z; Anal. Calcd for C
25H
29NO
3S: C, 70.89;
H, 6.90; N, 3.31; Found: C, 70.91; H, 6.95; N, 3.32.
7.
The checkers obtained
1 in
96% yield after recrystallization of the crude product
(see Note
8).
8.
(
1R, 2S)- and (
1S, 2R)-
1 exist as polymorphic
forms. Recrystallization from hot
ethyl acetate (4 mL/g of
1)
and
hexane (
ethyl acetate :
hexane
= 1 : 2) afforded higher melting crystals:
mp
124°C,
147-148°C, TLC
(silica gel) R
f = 0.52 in 3:1 hexanes :
ethyl acetate;
R
f = 0.22 in 9:1 hexanes :
ethyl acetate; (
1R,
2S)-
1
[α]D
23
+11.1° (c 2.24, CHCl3); (
1S, 2R)-
1:
[α]D
23 −11.2° (c 2.38,
CHCl3);
1H
NMR (500 MHz, CDCl
3) δ: 1.01 (t, 3 H, J = 7.4), 1.12
(d, 3 H, J = 7.0), 2.14 (m, 2 H), 2.27 (s, 3 H),
2.51 (s, 6 H), 4.04 (dq, 1 H, J = 4.0, 7.0), 4.60
(1 H, A of ABq, J
AB = 16.6), 4.72 (1 H, B of ABq, J
AB
= 16.6), 5.84 (d, 1 H, J = 3.9), 6.87 (s, 2 H), 6.88-6.96
(m, 2 H), 7.13≈7.35 (m, 8 H)
.
13C NMR (125 MHz CDCl
3) δ:
8.5, 12.3, 20.6, 22.7, 27.1,
47.9, 56.5, 77.7, 125.6, 126.8,
127.1, 127.5, 128.1 (2C), 131.9,
133.2, 138.4, 138.5, 139.9, 142.3,
172.2
; IR (film from
CH
2Cl
2) cm
−1 : 2982, 1747,
1604, 1454, 1381, 1324, 1205,
1153, 1080, 1056, 1020, 859,
764, 730, 699, 659
;
HRMS-FAB Calcd for C
28H
34NO
4S [M+H]+, 480.2209
m/
z
; Found, 480.2186
m/
z
; Anal. Calcd for C
28H
33NO
4S:
C, 70.12; H, 6.93; N, 2.92. Found: C, 70.40; H, 6.97; N, 2.90.
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
2-(N-Benzyl-N-mesitylenesulfonyl)amino-1-phenyl-1-propyl propionate:
Benzenesulfonamide, 2,4,6-trimethyl-N-[1-methyl-2-(1-oxopropoxy)-2-phenylethyl]-N-(phenylmethyl)-
(14); [R-(R*,S*)]-, (187324-66-9), [S-(R*,S*)]-, (187324-67-0)
2-(N-Mesitylenesulfonyl)amino-1-phenyl-1-propanol:
Benzenesulfonamide, N-(2-hydroxy-1-methyl-2-phenylethyl)-2,4,6-trimethyl-,
[S-(R*,S*)]- (14); (187324-62-5)
(1R,2S)-(−)-Norephedrine:
Norephedrine
(8);
Benzeneethanol, α-(1-aminoethyl)-, [R-(R*,S*)]- (9);
(492-41-1)
Triethylamine (8);
Ethanamine, N,N-diethyl-
(8,9); (121-44-8)
Mesitylenesulfonyl chloride:
2-Mesitylenesulfonyl
chloride (8);
Benzenesulfonyl chloride, 2,4,6-trimethyl-
(9); (773-64-8)
2-(N-Benzyl-N-mesitylenesulfonyl)amino-1-phenyl-1-propanol:
Benzenesulfonamide, N-(2-hydroxy-1-methyl-2-phenylethyl)-2,4,6-trimethyl-N-(phenylmethyl)-
(14); [R-(R*,S*)]-, (187324-63-6), [S-(R*,S*)]-, (187324-64-7)
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved