Org. Synth. 2008, 85, 172
DOI: 10.15227/orgsyn.085.0172
2-METHYLENECYCLOPROPANECARBOXYLIC ACID
[ETHYL ESTER]
Submitted by Mark E. Scott, Nai-Wen Tseng, and Mark Lautens
1a.
Checked by Pascal Dubé and John A. Ragan
1b.
1. Procedure
A. 2-Bromo-2-methyl-cyclopropanecarboxylic acid ethyl ester (1). A flame-dried, two-necked, 50-mL round-bottomed flask containing a magnetic stirring bar is fitted with septa to prevent exposure to air. Needles are then used for both an argon inlet and gas outlet to a bubbler. To this flask is added Rh2(OAc)4 (47.4 mg, 0.214 mmol Rh, 0.2 mol%) (Note 1) followed by 2-bromopropene (18.5 mL, 25.2 g, 0.208 mol, 2.18 equiv) (Note 2) by syringe. The argon inlet is removed and ethyl diazoacetate (11.0 mL, 10.6 g, 92.5 mmol, 1.00 equiv) (Note 3) is added by syringe pump over a period of two days (Note 4). Once the addition is complete, the reaction mixture is stirred for an additional 8 h (Note 5). The gas outlet is then removed and one septum is replaced with a one-piece, vacuum-jacketed short path distillation head containing an 18 mm (length) × 13 mm (OD) Vigreaux column. The excess 2-bromopropene is removed by distillation (760 mmHg, bp 42-45 °C) (Note 6). The reaction flask is cooled to room temperature, vacuum is applied (18–22 mmHg), and the flask is gradually heated in an oil bath to a bath temperature of 80 °C. This distillation provides 15.3-18.0 g (80-94% yield) of the bromo ester 1 (bp 64–73 °C, 18–22 mmHg) as a clear, colorless liquid (Notes 7–9).
B. 2-Methylene-cyclopropanecarboxylic acid, ethyl ester (2). A flame-dried, two-necked, 250-mL round-bottomed flask containing a magnetic stirring bar is fitted with a septum and a condenser containing a septum. An argon inlet and gas outlet is introduced through the condenser septa using needles. Under argon atmosphere, sodium hydride (6.10 g, 0.153 mol, 1.77 equiv) (Note 10) and diethyl ether (110 mL) (Note 11) are added. Bromo ester 1 (17.9 g, 0.0864 mol, 1.00 equiv) is then added via cannula using 10 mL of diethyl ether to complete the transfer. The argon inlet needle is removed, leaving the reaction solution vented to a bubbler. The reaction mixture is brought to reflux (oil bath set to 45 °C) before ethanol (1.1 mL, 0.019 mol, 0.22 equiv) (Note 12) is added (CAUTION: addition of the ethanol should be done slowly over the course of several minutes to prevent excessive hydrogen gas evolution). The resulting, light-brown solution is stirred at reflux for 15 h (reaction progress can be monitored by GC-MS, Note 13). The reaction mixture is then cooled and the precipitated tan solid is filtered through a 1 cm pad of Celite® in a sintered glass Büchner funnel (8.5 cm ID). The residue is washed with diethyl ether (2 × 50 mL) and the resulting yellow filtrate is distilled at 760 mmHg using a 12 cm (length) × 17 mm OD Vigreaux column and short path distillation apparatus (18 mm (length) × 13 mm (OD)) to remove the diethyl ether. Vacuum is then applied (13–14 mmHg) and the resulting yellow oil residue is then distilled to afford 7.75 g (71% yield) of 2-methylenecyclopropane carboxylic acid ethyl ester (2) (40–44 °C, 13–14 mmHg) as a clear, colorless liquid. A second distillation (Note 14), necessary to remove a trace impurity, is performed to provide 7.28 g (66% yield) of analytically pure product (Note 15).
2. Notes
1.
Rhodium acetate dimer was purchased from Strem Chemicals, Inc. and was used as received.
2.
2-Bromopropene (99%, stabilized with copper) was purchased from Alfa Aesar and was stored at 5 °C until it was used as received.
3.
Ethyl diazoacetate (≤ 15% dichloromethane) was purchased from Aldrich and was stored at 5 °C until it was used as received.
1H NMR analysis found that the dichloromethane content was 15 mol% = 12 wt%. This value was used to determine the mass of reagent added to the reaction = (11.0 mL)×(1.085 g/mL)×(0.85 mol × 114.1 g/mol) / [(0.85 mol × 114.1 g/mol) + (0.15 mol × 84.93 g/mol)] = 10.55 = 10.6 g. The density for ethyl diazoacetate (
d = 1.085) was used for determining the mass of the starting material.
4.
A KD Scientific (model 100) syringe pump was used to deliver the ethyl diazoacetate over a period of two days at approximately 0.20 mL/hour.
5.
The submitters stirred the reaction mixture for 3 hours after complete addition. In principle this additional time is unnecessary.
6.
Approximately
8.8 g of 2-bromopropene containing approximately 14% by mass of dichloromethane (by
1H NMR) could be recycled from this reaction. Periodic cooling using a dry ice/acetone bath should also be used in order to prevent evaporation of the 2-bromopropene mixture in the receiving flask.
7.
GC analysis (see
Note 13 for method) revealed that the product mixture was >98% pure (trans:cis ratios ranged from 1.5-2.0:1.
tR(trans), 4.04 min;
tR(cis), 4.56 min). The product exhibited the following physicochemical properties: IR (film) cm
−1: 2980, 1725, 1378, 1267, 1184, 1147, 1088, 1056, 854;
1H NMR
pdf (400 MHz, CDCl
3) δ: 1.11 (dd, 1 H cis,
J = 8.7, 6.0 Hz), 1.17 (t, 3 H trans,
J = 7.5 Hz), 1.19 (t, 3 H cis,
J = 7.5 Hz), 1.31 (t, 1 H trans,
J = 6.6 Hz), 1.47 (dd, 1 H trans,
J = 9.6, 6.7 Hz), 1.59-1.70 (m, 2 H cis), 1.72 (s, 3 H cis), 1.74 (s, 3 H trans), 2.18 (dd, 1 H trans,
J = 9.5, 7.0 Hz), 4.04 (q, 2 H trans,
J = 7.1 Hz), 4.09 (dq, 2 H cis,
J = 7.1, 1.3 Hz);
13C NMR
pdf (100 MHz, CDCl
3) δ: 14.0, 14.1, 22.6, 23.6, 24.0, 28.4, 29.3, 30.5, 32.7, 32.9, 60.7, 60.8, 168.8, 169.8. Anal. Calcd for C
7H
11O
2Br: C, 40.60; H, 5.35. Found: C, 40.39; H, 5.44.
8.
In this case, the isomers refer to the relationship between the bromine and ester moieties. ROESY analysis of the mixture in benzene-
d6 was used to determine the configuration for both isomers.
9.
The checkers found that it was important to maintain sufficient vacuum during the distillation (ca. 15 mmHg) to keep the temperature near the 40–44 °C cited in the procedure in order to avoid decomposition of the product.
10.
Sodium hydride (60% in mineral oil) was purchased from Aldrich and used as received.
11.
The checkers used BHT-stabilized, anhydrous diethyl ether purchased from JT Baker, which was used as received. The submitters used diethyl ether distilled from Na/benzophenone immediately prior to use.
12.
The checkers used ethanol purchased from Pharmco-AAPER (200 proof, ACS grade), which was used as received. The submitters used ethanol that was distilled from magnesium/iodine and was kept over 4Å molecular sieves under argon until used.
13.
GC/MS analysis was performed on an HP 6890 Series GC coupled with an Agilent 5973 mass spectrometer with an HP-1 column (0.2 mm × 12 m, 0.33 μm film thickness). A mass spec (EI) detector was utilized with a 1 min solvent delay. Injection size was 1 μL. Inlet was in split mode, with an injection temperature of 250 °C, flow rate (He) of 1.0 mL/min, and a column gradient from 50 °C for 30 seconds, then ramp at a rate of 10 °C/min to 275 °C and hold for 1 min. Samples for GC analysis were prepared by removing a 10 μL aliquot from the reaction and diluting to 1 mL with acetonitrile.
14.
The second distillation may not be necessary depending on the intended use for the product.
15.
GC analysis (see
Note 13 for method) revealed that the product was >98% pure (
tR, 2.19 min) and exhibited the following physicochemical properties: IR (film) cm
−1: 2982, 1732, 1446, 1370, 1333, 1296, 1262, 1177, 1110, 1050, 906, 720;
1H NMR
pdf (400 MHz, CDCl
3) δ: 1.16 (t, 3 H,
J = 7.0 Hz), 1.50-1.55 (m, 1 H), 1.69-1.72 (m, 1 H), 2.13-2.16 (m, 1 H), 4.04 (q, 2 H,
J = 7.0 Hz), 5.40-5.42 (m, 2 H);
13C NMR
pdf (100 MHz, CDCl
3) δ: 11.1, 13.9, 17.8, 60.4, 104.2, 130.1, 171.7; Anal. Calcd. for C
7H
10O
2: C, 66.65; H, 7.99. Found: C, 66.49; H, 7.69.
Handling and Disposal of Hazardous Chemicals
The procedures in this article are intended for use only by persons with prior training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011 www.nap.edu). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
These procedures must be conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
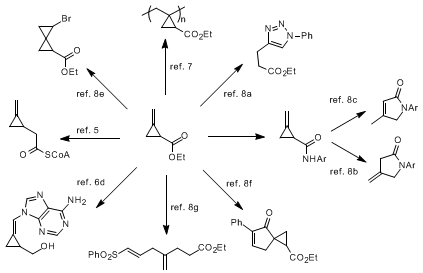
3. Discussion
Previous methods to prepare this versatile methylenecyclopropane building block have been reported in low yields via the cyclopropanation of either 2-bromopropene (copper-catalyzed)
2 or ethyl buta-2,3-dienoate (Simmons-Smith cyclopropanation).
3 A subsequent report for the preparation of this methylenecyclopropylcarboxylic acid ethyl ester was reported in much higher yields using a rhodium diacetate catalyst.
4 Although this latter report was higher yielding, yields for this process were sometimes variable due to the volatility of the product and intermediates. This approach removes any unnecessary manipulation steps, thereby preventing possible loss due to evaporation while still obtaining high chemical yields of the product. In addition, this modified method allows for the reuse of approximately one third of the unused starting material, making this method highly cost efficient.
The resulting 2-methylenecyclopropanecarboxylic acid ethyl ester has been extensively used for the preparation of model compounds for the study of the hypoglycemic activity of methylenecyclopropylglycine,
4,5 and for the synthesis of a variety of antiviral nucleoside analogues.
6 In addition, the methylenecyclopropanecarboxylic acid ethyl ester has also been shown to be a useful reagent for the synthesis of functionalized polymers containing a highly strained cyclopropane moiety in the polymer backbone.
7 A summary of these applications, as well as additional synthetic transformations
8 from either the ester or its derivatives, are detailed below.
Appendix
Chemical Abstracts Nomenclature (Collective Index Number);
(Registry Number)
Rhodium acetate dimer:
Rhodium, tetrakis[μ-(acetato-κO:κO')]di-, (Rh-Rh); (15956-28-2)
2-Bromopropene:
1-Propene, 2-bromo-; (557-93-7)
Ethyl diazoacetate:
Acetic acid, 2-diazo-, ethyl ester; (623-73-4)
Sodium hydride; (7646-69-7)
Cyclopropanecarboxylic acid, 2-bromo-2-methyl-, ethyl ester; (89892-99-9)
Cyclopropanecarboxylic acid, 2-methylene-, ethyl ester; (18941-94-1)
 |
Mark Scott was born in Simcoe, Ontario, Canada. He received his Bachelor's degree (Honours) in Engineering Chemistry and later his M.Sc.Eng. in Chemical Engineering from Queen's University under the supervision of Profs. Scott Parent and Ralph Whitney. Following his Master's, he received his Ph.D. in Mark Lautens' research group at the University of Toronto where he studied Lewis acid-mediated ring expansions of methylenecyclopropanes. He has been the recipient of NSERC Postgraduate (M.Sc.Eng. and Ph.D.) Scholarships. He is currently an NSERC postdoctoral fellow at Princeton University under Prof. David MacMillan.
|
 |
Nai-Wen Tseng was born in Taipei, Taiwan in 1979. He received a Bachelor of Science degree in Chemistry at the National Tsing Hua University in 2001 where he conducted his undergraduate research under Prof. Hsing-Jang Liu. He is currently pursuing his Ph.D. degree under the guidance of Prof. Mark Lautens at the University of Toronto. His research is focused on the development of new rhodium-catalyzed addition/cyclization reactions.
|
 |
Pascal Dubé was born in 1978 in St-Pacôme, Québec, Canada. He received a B.Sc. degree in pharmaceutical chemistry in 2000 from Université de Sherbrooke. As part of a Co-Op program he did internships at Merck Frosst Inc. and Pfizer. He then received a Ph.D. from Université de Sherbrooke in 2005 under the guidance of Professor Claude Spino. His doctoral research involved the development of a synthetic route to quassinoids featuring the Diels-Alder cycloaddition of transmissible dienes. After a postdoctoral fellowship with Professor F. Dean Toste at UC Berkeley he joined the Pfizer Chemical Research and Development group in 2006.
|
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved