Org. Synth. 2023, 100, 84-98
DOI: 10.15227/orgsyn.100.0084
Synthesis of Diethyl (1-Diazo-2-oxopropyl)phosphonate
Submitted by Cholapat Varongchayakul and Rick L. Danheiser*
1Checked by Michael D. Clift, Steve M. Richter, Zhe Wang, and Seble Wagaw
1. Procedure (Note 1)
Diethyl (1-diazo-2-oxopropyl)phosphonate (1). A 1-L, three-necked, round-bottomed flask is equipped with a 125-mL pressure-equalizing addition funnel fitted with a rubber septum, an argon inlet adapter, a rubber septum, and a 50 x 20 mm Teflon-coated oval magnetic stir bar (Figure 1A) (Note 2). The flask is charged with NaH (1.04 g, 60% dispersion in mineral oil, 26 mmol, 1.0 equiv) (Notes 3 and 4) and toluene (50 mL) (Note 5). The stirred suspension of NaH is cooled to 0 °C in an ice-water bath, and a solution of diethyl (2-oxopropyl)phosphonate (5.05 g, 26 mmol, 1.0 equiv) (Note 6) in toluene (40 mL) is added dropwise via the addition funnel (Note 7) over 15 min. A white precipitate appears immediately accompanied by bubbling, which subsides after several minutes (Figure 1B). Toluene (10 mL) is charged to the addition funnel and then added dropwise over 2 min to wash any residual phosphonate solution into the reaction flask.
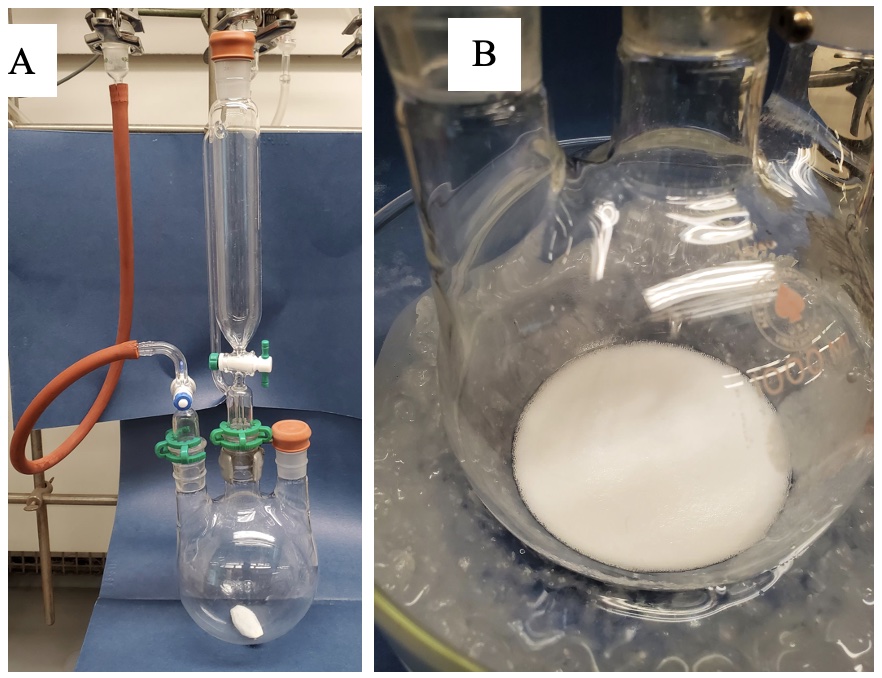
Figure 1. (A) Reaction assembly; (B) Reaction mixture after complete addition of diethyl (2-oxopropyl)phosphonate (photos provided by submitters)
After 10 min, a solution of 4-acetamidobenzenesulfonyl azide (6.25 g, 26 mmol, 1.0 equiv) in 40 mL of THF is added dropwise via the addition funnel over 15 min (Notes 9 and 10). Additional THF (10 mL) is then charged to the addition funnel and added dropwise over 2 min to wash any residual azide solution into the reaction mixture. The ice-water bath is then removed and the reaction mixture is allowed to stir at room temperature for 20 h (Figures 2A-2C). The resulting orange mixture is diluted with hexanes (25 mL) (Note 11) and filtered under vacuum (20 mmHg) through a pad of Celite (20 g) (Note 12) in a 500-mL sintered glass filter funnel into a 1-L Erlenmeyer flask (Figures 3A-3B). The material in the funnel is washed with methyl t-butyl ether (3 x 20 mL) (Note 13). The filtrate is next transferred to a 1-L, one-necked, round-bottomed flask (Figure 3C) with the aid of three 10-mL portions of methyl t-butyl ether and then concentrated by rotary evaporation (25 °C, 10 mmHg) to yield a pale-yellow oil (7.71 g) (Figure 4) (Note 14).
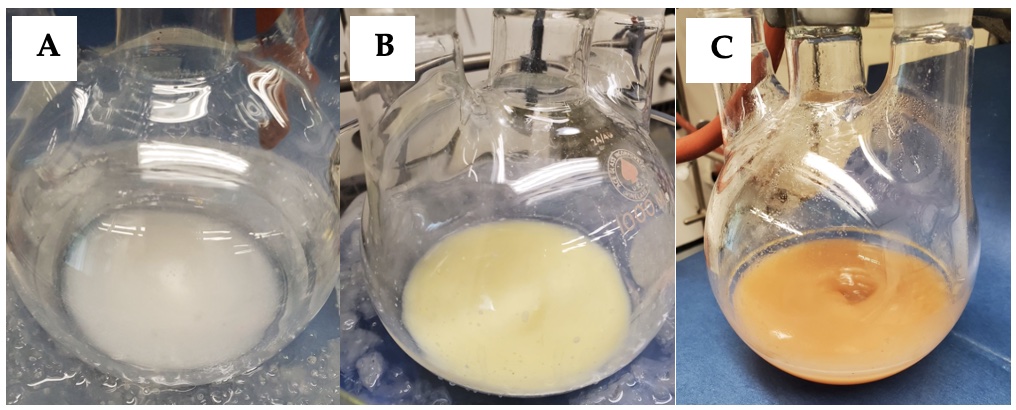
Figure 2. (A) Reaction mixture before addition of 4-acetamidobenzenesulfonyl azide; (B) Reaction mixture after addition of 4-acetamidobenzenesulfonyl azide; (C) Reaction mixture after 20 h (photos provided by submitters)
Figure 3. (A) Filtration set up; (B) Precipitate obtained; (C) Filtrate containing crude reaction mixture in a 1-L round-bottomed flask (photos provided by submitters)
This material is purified by chromatography on silica (Notes 15 and 16) using 1:1 ethyl acetate-hexanes (Notes 11 and 17). Fractions containing the product are concentrated by rotary evaporation (25 °C, 10 mmHg). Further drying under vacuum (25 °C, 0.05 mmHg) yields 5.17 g (90%) of 1 as a pale-yellow oil (Figure 5B) (Notes 18, 19, 20, 21, and 22).
Figure 4. Crude product (photo provided by submitters)
Figure 5. (A) Fractions containing product; (B) Diethyl (1-diazo-2-oxopropyl)phosphonate (photos provided by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
sodium hydride,
diethyl (2-oxopropyl)phosphonate,
4-acetamidobenzenesulfonyl azide,
hexanes,
tetrahydrofuran,
methyl tert-butyl ether,
ethyl acetate,
toluene, and silica gel.
Differential Scanning Calorimetry (DSC) studies on the reagents and product were performed by the checkers, and the results are summarized in the appendix.
2. Glassware was flame-dried under vacuum (0.1 mmHg), back-filled with argon while hot, and then maintained under the inert atmosphere during the course of the reaction. The checkers did not flame-dry the glassware and obtained similar results reported by the submitters. The relatively large stir bar is required for efficient stirring of the heterogeneous reaction mixture once the
4-acetamidobenzenesulfonyl amide byproduct precipitates from solution. Use of an overhead stirrer is a recommended alternative.
3.
Sodium hydride (60% by weight dispersion in mineral oil) was purchased from Oakwood Chemicals and used as received.
NaH was weighed into a 20-dram vial and then added to the reaction flask via a powder funnel. The vial was rinsed with 10 mL of
toluene. An additional 40 mL of
toluene was charged into the reaction flask by syringe resulting in a total of 50 mL of
toluene.
4. The use of impure samples of
NaH (contaminated with
NaOH formed during storage) led to reduced yields and product contaminated with
DAMP (
diethyl diazomethylphosphonate) presumably generated by cleavage of the product by hydroxide.
DAMP is not easily separated from the product by chromatography; the product (
1) has R
f = 0.24 (silica gel, elution with 1:1
ethyl acetate-
hexanes) while
DAMP gives a slightly more polar overlapping spot.
5. Anhydrous
toluene (Sigma-Aldrich, 99.8%) was used without purification by the checkers.
6.
Diethyl 2-(oxopropyl)phosphonate (96%) was purchased from Sigma-Aldrich and used it as received.
7. A solution of
diethyl (2-oxopropyl)phosphonate in 30 mL of
toluene was prepared in an oven-dried 50-mL, one-necked, round-bottomed flask and transferred to the addition funnel under argon via cannula (Figure 6). The flask was washed with two 5-mL portions of
toluene which are cannulated into the addition funnel.
Figure 6. Cannulation set up (photo provided by submitters)
8.
4-Acetamidobenzenesulfonyl azide (97%) was purchased from Oakwood Chemicals and used as received.
9. Anhydrous
tetrahydrofuran (>99% with 250 ppm BHT) was purchased from Sigma-Aldrich and used without purification.
10. A solution of
4-acetamidobenzenesulfonyl azide in 30 mL of
THF was prepared in an oven-dried 50-mL, one-necked, round-bottomed flask and transferred to the addition funnel under argon via cannula. The flask was washed with two 5-mL portions of
THF which are cannulated into the addition funnel.
11.
Hexanes (mixture of isomers, >99.7%) was purchased from Sigma-Aldrich and used as received.
12. Filtration through Celite is required to avoid the clogging observed when filtration of the sulfonamide byproduct is attempted using filter paper or a sintered glass funnel. Celite was purchased from EMD Chemicals and used as received.
13.
Methyl tert-butyl ether (ACS Grade, >99.0%)) was purchased from Sigma-Aldrich and used as received.
14. A second run on identical scale yielded 5.85 g of a pale, yellow oil.
15. Purification was performed using automated column chromatography by the following procedure. The crude product was loaded onto a 40-mm diameter column packed with silica gel (40 g) compatible with CombiFlash Nextgen 100. Elution was carried out with 1:1
ethyl acetate/
hexanes (Notes
11 and
17), and 18-mL fractions were collected in 18 x 160 mm test tubes. (Figure 7)
Figure 7. Chromatogram from automated chromatographic separation using CombiFlash Nextgen 100 (photo provided by submitters)
16. Alternatively, the crude product was loaded onto a 60-mm diameter column packed with silica gel (60 g, VWR "Industrial Grade" irregular silica gel 60A, 40-60 μm, 200-300 mesh) that was prepared as a slurry in 1:1
ethyl acetate-
hexanes (Notes
11 and
17). Elution was performed with 1:1
ethyl acetate-
hexanes, and 20-mL fractions were collected in 18 x 160 mm test tubes with the presence of the desired product monitored by TLC (R
f = 0.24 (1:1
ethyl acetate-
hexanes; J.T. Baker silica gel plate). The product was collected in fractions 11-46, which were combined in a 2-L round-bottomed flask (Figure 5A) and evaporated to dryness using a rotary evaporator (25 °C, 10 mmHg).
17.
Ethyl acetate (>99.7%) was purchased from Sigma-Aldrich and used as received.
18. The submitters report that a slightly higher yield of
1 (5.32 g, 93%, 97% wt purity) can be obtained by increasing the amount of
diethyl 2-(oxopropyl)phosphonate to 1.1 equiv (5.55 g, 28.6 mmol).
19.
Diethyl (1-diazo-2-oxopropyl)phosphonate (
1) has the following physical and spectroscopic properties: R
f = 0.24 (1:1
ethyl acetate-
hexanes; J.T. Baker silica gel plate);
1H NMR
pdf (CDCl
3, 400 MHz) δ: 4.31-4.10 (m, 4H), 2.29 (s, 3H), 1.40 (td, 6H).;
13C NMR
pdf (CDCl
3, 100 MHz) δ: 190.1 (d,
J = 13.1 Hz), 65.6, 63.4 (d,
J = 6.1 Hz), 27.2, 16.1 (d,
J = 6.1 Hz).;
31P NMR
pdf (CDCl
3, 162 MHz) δ: 11.02; FTIR (neat) (cm
-1) 2986, 2117, 1655, 1260, 1006. The
13C resonance of the carbon bearing the diazo group, which appears at 65.6 ppm, is likely one peak of a doublet, with the other peak of the doublet buried beneath the resonance at 63.4 ppm. The submitters report the diazo carbon's
13C resonance as 64.1 (d,
J = 217 Hz); note, however, that some prior reports do not observe this resonance due to long carbon T1 relaxation times.
20.
Diethyl (1-diazo-2-oxopropyl)phosphonate (
1) is stable to storage neat under air for at least 3 months.
21. The purity of product (
1) was determined by qNMR analysis on a sample prepared by dissolving 16.8 mg of
1 and 15.1 mg of
1,3,5-trimethoxybenzene (99.9%) in 1.0 mL CDCl
3 in a 1 dram vial. The purity was determined to be 97wt%.
22. A second run on identical scale provided 4.97 g (87%) of
1 as a pale-yellow oil.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Diethyl and dimethyl (1-diazo-2-oxopropyl)phosphonate
1 and
2 (Scheme 1) are versatile building blocks for organic synthesis.
2 These diazo compounds react via dipolar cycloaddition and other pathways to provide access to diverse classes of nitrogen heterocycles including phosphoryl-substituted pyrazoles, triazolines, oxazoles, and thiazoles.
2 In 1989, Ohira
3 showed that cleavage of the acetyl group can be effected under mildly basic conditions, providing a convenient method for the generation of the Seyferth-Gilbert reagent (diazomethyl)phosphonate ("DAMP")
4 in situ for further transformations. Colvin had previously shown that DAMP reacts with carbonyl compounds in the presence of base to form vinylidene carbenes which are useful synthetic intermediates.
5 Vinylidene carbenes derived from aldehydes rearrange smoothly to alkynes,
5,6 while those generated from ketones can be trapped by alcohols to form enol ethers.
3,7Scheme 1. Diethyl and dimethyl (1-diazo-2-oxopropyl)phosphonate
The application of
1 and
2 for the one-carbon homologation of aldehydes to alkynes was first reported by Ohira
3 and subsequently developed further by Bestmann.
8 Today the "Ohira-Bestmann reaction" provides one of the most widely used methods for the synthesis of acetylenes.
9 Although (diazomethyl)phosphonate (DAMP) itself is commercially available, DAMP is expensive and has limited shelf life, and the Ohira-Bestmann protocol provides an efficient and convenient means of generating the conjugate base of DAMP in situ for reaction with aldehydes.
Although commercially available, both diethyl and dimethyl (1-diazo-2-oxopropyl)phosphonate are relatively expensive and so many labs opt to prepare the reagents which can then be stored indefinitely for use in the Ohira-Bestmann homologation and other reactions. As summarized in Scheme 2, several groups have reported the application of diazo transfer
10 to dialkyl (2-oxopropyl)phosphonate as an efficient method for the preparation of the "Ohira-Bestmann reagents"
1 and
2.
11,12,13,14,15Vandewalle described the first synthesis of
2 via diazo transfer in 1984 using TsN
3.
11 The hazards associated with this azide led Meffre
12 and Pietruszka
14 to instead employ
4-acetamidobenzenesulfonyl azide ("
p-ABSA"), a sulfonyl azide introduced by Davies
16 in 1987 as a safer alternative to MsN
3 and TsN
3. Finally, Hanson
13 has deployed a polymeric sulfonyl azide to facilitate separation of the sulfonamide byproduct formed in the reaction, and Kristensen
15 has described the use of an imidazolyl sulfonyl azide for the generation of
2 in situ for reactions with aldehydes in the Ohira-Bestmann alkyne synthesis.
The procedure described in this article employs a modification of the method of Pietruszka.
14 We have found that
sodium hydride is a reliable choice as base for the reaction provided that it is not contaminated with
NaOH formed by exposure to moisture during storage. Reduced yields of
1 were obtained using
NaH from old containers due to cleavage of the acetyl group by nucleophilic hydroxide. Attempts to effect the diazo transfer using DBU in place of
NaH led to complex mixtures and reaction with Et
3N was very slow and afforded <10% of the desired diazo compound.
Scheme 2. Synthesis of the Ohira-Bestmann reagents 1 and 2
As the diazo transfer reagent, we favor the use of p-ABSA which is commercially available, stable to storage, and safer to handle as compared to other sulfonyl azide reagents. In addition, the sulfonamide byproduct formed in the reaction of this azide can be separated by filtration, simplifying the purification of the diazo product by chromatography.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
Diethyl (2-oxopropyl)phosphonate; (1067-71-6)
Sodium hydride; (7646-69-7)
4-Acetamidobenzenesulfonyl azide; (2158-14-7)

|
Cholapat Varongchayakul was born in Chonburi, Thailand in 2001. He is currently an undergraduate at the Massachusetts Institute of Technology and expects to receive his B.S. degree in Chemistry in 2024. Cholapat joined the laboratory of Professor Danheiser as an undergraduate researcher in 2020. |

|
Rick L. Danheiser received his undergraduate education at Columbia University where he carried out research in the laboratory of Professor Gilbert Stork. He received his Ph.D. at Harvard University in 1978 working under the direction of E. J. Corey on the total synthesis of gibberellic acid. Dr. Danheiser is the A. C. Cope Professor of Chemistry at MIT where his research focuses on the design and invention of new annulation and cycloaddition reactions, and their application in the total synthesis of biologically active compounds. |

|
Mike Clift is a Principal Research Scientist in the Process Chemistry group at AbbVie. He received his B.S. from Western Michigan University and his Ph. D. from Northwestern University (NU). At NU, he worked with Regan Thomson to develop synthetic methods that were leveraged towards the synthesis of several alkaloid natural products. Mike then moved to Princeton University where he developed a photoredox-organocatalysis platform for C-H functionalization as a post-doctoral scholar with Dave MacMillan. Mike started his independent career at The University of Kansas, where his group developed methods for the synthesis and functionalization of N-containing compounds. Mike joined AbbVie in 2019. |

|
Steve Richter has been in AbbVie Process Engineering for 19 years. He received his BChE from Georgia Tech and his Ph.D. from Purdue University, both in chemical engineering. Since joining industry, Steve has worked process safety and reaction engineering groups in the pharmaceutical and specialty chemical processes. |

|
Zhe Wang has been in Abbott/AbbVie Process Safety Laboratory for 25 years. He received his B.S. from Tianjin University in China and his Ph.D. from Northwestern University, both in Chemical Engineering. While at Northwestern, he studied multiphase flow under Professor George Bankoff. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved