Org. Synth. 2023, 100, 186-198
DOI: 10.15227/orgsyn.100.0186
Synthesis of Enantioenriched NH-Sulfoximines by NH Transfer to Sulfoxides Using Ammonium Carbamate and (Diacetoxyiodo)benzene
Submitted by Edward L. Briggs, Tsz-Kan Ma, Zhenhao Zhong, Arianna Tota, Leonardo Degennaro, Renzo Luisi* and James A. Bull*
1Checked by Ellie Plachinski and Tehshik Yoon
1. Procedure (Note 1)
(S)-(4-Bromophenyl)(imino)(methyl)-λ6-sulfanone (2). In air, a 250 mL one-necked round-bottomed flask (24/40), equipped with a Teflon coated oval-shaped magnetic stirrer bar (40 mm × 20 mm), is suspended in a water bath at 25 °C and charged with MeOH (70 mL) (Note 2). Stirring of 250 rpm is initiated. (S)-(-)-4-Bromophenyl methyl sulfoxide (1) (7.67 g, 35.0 mmol, 1.0 equiv) (Notes 3 and 4) followed by (diacetoxyiodo)benzene (33.8 g, 105 mmol, 3.0 equiv) (Note 5) are added to the flask as single portions via powder funnel. Ammonium carbamate (10.9 g, 140 mmol, 4.0 equiv) (Note 6) is then added gradually to the suspension in roughly ten equal portions over a period of ten min allowing for controlled decarboxylation. The reaction mixture is stirred at 25 °C for 1 h, during which time the reaction mixture turns from colorless to yellow, and reaction progress is monitored by TLC (Figure 1a and b) (Note 7).
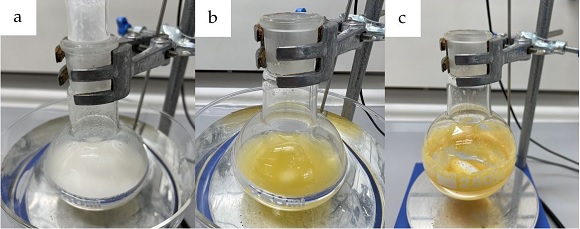
Figure 1. a) Decarboxylation of ammonium carbamate; b) Reaction mixture after 1 h; c) Reaction mixture after removal of methanol (photos provided by submitters)
The organic solvent is evaporated under reduced pressure (25 ºC, 35 mmHg) to give a yellow slurry (Figure 1c), which is diluted with EtOAc (150 mL) (Note 8) and saturated aqueous NaHCO3 (100 mL). The mixture is stirred for 5 min to neutralize the acetic acid formed during the reaction. The resulting biphasic solution is transferred to a 1 L separatory funnel, rinsing the flask with EtOAc (50 mL) that is added to the separatory funnel. The layers are separated, and the organic layer is washed with distilled H2O (100 mL) to remove residual ammonium carbamate. The organic layer is extracted with aqueous 1 M HCl (3 × 100 mL) (Figure 2a). The combined aqueous layers are back extracted with EtOAc (3 x 100 mL). These three extracts are combined and washed with distilled H2O (100 mL), after which the organic layer is extracted with HCl (1 M; 3 x 50 mL). Phases are separated and the separatory funnel is rinsed with EtOAc (2 × 100 mL), which is discarded. The combined acidic aqueous fractions are transferred back into the separatory funnel and washed with CH2Cl2 (2 × 100 mL) (Note 9), which is removed. The aqueous layer is then basified with aqueous NaOH (2 M; 200 mL). The basified aqueous layer is extracted with CH2Cl2 (4 × 100 mL). The combined organic fractions are dried over anhydrous Na2SO4 (ca. 20 g) (Note 10). The solution is filtered through a 7 cm glass funnel plugged with cotton wool, and the Na2SO4 is washed with CH2Cl2 (50 mL) into a 500 mL round-bottomed flask (Figure 2b). The organic solvent is evaporated under reduced pressure (25 °C, 250 mmHg) and the flask is then placed under high vacuum (21 °C, 0.1 mmHg) for 18 h to remove any residual solvent to yield (S)-(4-bromophenyl)(imino)(methyl)-λ6-sulfanone (2) as a white solid (5.50 g, 66%, 99% purity, 99% ee) (Figure 2c) (Notes 11, 12 and 13).
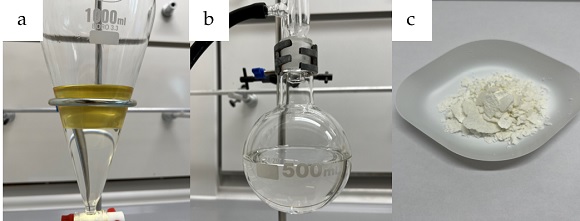
Figure 2. a) Extraction using aqueous HCl (1 M); b) Product in CH2Cl2 after work up; c) Final compound 2 (photos provided by submitters)
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
methanol,
(S)-(-)-4-bromophenyl methyl sulfoxide (
1),
ammonium carbamate,
(diacetoxyiodo)benzene,
dichloromethane,
sodium sulfate, silica gel,
ethyl acetate,
hexane, and
(S)-(4-bromophenyl)(imino)(methyl)-λ6-sulfanone (
2).
2. HPLC grade
methanol (99.8%) was purchased from Fisher Scientific and used as received.
3.
(S)-(-)-4-Bromophenyl methyl sulfoxide (
1) (98.0%) was synthesized according to a previously described
Organic Syntheses article from Jackson and co-workers.
2 Two batches on a 60 mmol scale were run as described up to the point of recrystallisation. The two batches were combined and recrystallized to afford the desired starting sulfoxide in a combined yield of 60% and 98%
ee.
2 The enantiomeric excess was determined to be 98% by chiral HPLC on Chiralpak IE column using
hexanes/
isopropyl alcohol (90:10) at a flow rate of 1.0 mL/min, while monitoring at 254 nm. The retention time (tR) of the major enantiomer = 24.3 min, and the retention time (tR) of the minor enantiomer = 26.5 min.
4. Procedure for preparing the racemic starting material: To a solution of
p-bromophenyl methyl sulfide (15.3 g, 75 mmol, 1.0 equiv) in
acetic acid (15.0 mL, 253 mmol, 3.4 equiv) was added 30 wt%
H2O2 in
H2O solution (7.7 mL, 75 mmol, 1.0 equiv) at 0 °C and allowed to warm to room temperature. After 2 h the reaction was quenched at 0 °C with 1 M
NaOH (150 mL) and the aqueous mixture was extracted with
CH2Cl2 (3 ×150 mL). The combined organic layers were dried (
Na2SO4), filtered and the solvent was removed under reduced pressure to afford the desired product as a white solid (16.4 g).
5.
(Diacetoxyiodo)benzene (98%) was purchased from Sigma-Aldrich and used as received.
6.
Ammonium carbamate (99%) was purchased from Sigma-Aldrich and used as received.
7. Reaction progress can be monitored by silica gel TLC analysis with 40%
acetone in
hexane using
KMnO4 as a stain (Figure 3). TLC Silica gel 60 F254 glass backed plates were purchased from Merck, Inc. The starting material
1 has a R
f = 0.31 and the product
2 has a R
f = 0.19.
Figure 3. Thin layer chromatographic (TLC) analysis of the reaction (photos provided by submitters)
8.
Ethyl acetate (99.5%) was purchased from Sigma-Aldrich and used as received.
9.
Dichloromethane (99.9%) was purchased from Sigma-Aldrich and used as received.
10. Anhydrous
sodium sulfate (99%) was purchased from Fisher Scientific and used as received.
11. A second reaction on half scale provided 3.25 g (79%, 99% purity, 92%
ee) of the desired product.
12. The purity of the compound was calculated by quantitative
1H NMR
pdf analysis with a relaxation delay of 30 sec using 20.4 mg of
1,3,5-trimethoxy-benzene (purity 98%) and 33.9 mg of compound
2.
13. The enantiomeric excess was determined to be 99% by chiral HPLC on Chiralpak IE column using
hexanes/
isopropyl alcohol (90:10) at a flow rate of 1.0 mL/min, while monitoring at 254 nm. Retention time (tR) of the major enantiomer = 39.5 min, and retention time (tR) of the minor enantiomer = 44.4 min. Analytical data
3 for
(S)-(4-bromophenyl)-(imino)(methyl)-λ6-sulfanone (
2): mp = 104-105 ºC.
1H NMR
pdf (500 MHz, CDCl
3) δ: 7.88 (d,
J = 8.8 Hz, 2H), 7.70 (d,
J = 8.7 Hz, 2H), 3.10 (s, 3H), 2.69 (s, 1H).
13C NMR
pdf (126 MHz, CDCl
3) δ: 142.7, 132.6, 129.4, 128.3, 46.3. IR (film) 3304, 3278, 2926, 1570, 1382, 1218, 1090, 1063, 943, 759, 511 cm
-1 . [α]
D 23= +20.0 (c = 0.97, CHCl
3). (lit.
4 [α]
D25 = +17.8 (c = 0.93, CHCl
3)).
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Sulfoximines are emerging motifs in the life sciences and have shown application in asymmetric synthesis as chiral auxiliaries, ligands and catalysts.
5 Their Lewis basic character has also made them interesting directing groups for C-H functionalization.
6 Their most promising applications are in the pharmaceutical and agrochemical industries as bioisosteres for sulfones and sulfonamides.
7 Recent years have seen broad uptake in medicinal chemistry and several examples of sulfoximines in clinical candidates.
7b,8 Until relatively recently, uptake in the life sciences had been limited by the lack of safe and amenable synthetic methods, which have seen significant recent development.
9,10 In 2016 we reported an operationally simple, metal-free protocol for the preparation of NH-sulfoximines from sulfoxides, using
ammonium carbamate as a convenient source of ammonia and (diacetoxyiodo)benzene as the oxidant.
11 We (and others) later reported the synthesis of NH-sulfoximines directly from sulfides under similar conditions,
12,13 as well as the synthesis of sulfonimidamides.
14,15The described NH-transfer to sulfoxides to form sulfoximines proceeds stereospecifically.
11 As such the method described provides an effective means of accessing highly enantioenriched sulfoximines from enantioenriched sulfoxides, with complete retention of
ee observed. A broad reaction scope has been demonstrated, that has been shown to be tolerant of polar functionality and pyridine-type heterocycles. The mechanism is proposed to proceed through the initial condensation of ammonia with the hypervalent iodine reagent leading to an iminoiodinane which undergoes oxidation to form a highly reactive iodonitrene. The iodonitrene reacts directly with the sulfoxide to form the protected sulfoximine with the breaking of the N-I bond occurring during removal of the reaction solvent. For evidence of these mechanistic steps see the original report,
11 as well related reports on sulfides and sulfenamides.
12,14Overall, we expect this metal-free protocol from enantioenriched sulfoxides to be broad applicable, with the demonstrated compound presenting an attractive building block for further derivatization.
Appendix
Chemical Abstracts Nomenclature (Registry Number)
(S)-(-)-4-Bromophenyl methyl sulfoxide: Benzene, 1-bromo-4-[(S)-methylsulfinyl]-; (1) (145166-15-0)
Ammonium carbamate: Carbamic acid, ammonium salt (1:1); (1111-78-0)
(Diacetoxyiodo)benzene: Iodine, bis(acetato-κO)phenyl-; (3240-34-4)
(S)-(4-Bromophenyl)(imino)(methyl)-λ6-sulfanone: Sulfoximine, S-(4-bromophenyl)-S-methyl-, [S(S)]-; (2) (2258639-09-5)
Methanol; (67-56-1)

|
Edward Briggs obtained his MChem Chemistry with six-month placement in 2017 from the University of Southampton. During this time, he worked with Professor Bruno Linclau on the fluorination of glucose and galactose derivatives, as well as undertaking a placement at Vertex Pharmaceuticals where he worked simultaneously on bespoke reagent synthesis and the lead medicinal chemistry project. Edward started his Ph.D. with Dr James Bull at Imperial College London in October 2017 looking at novel synthetic methods to access sulfoximines and sulfonimidamides. |

|
Tsz-Kan Ma received his M.Sci. in Chemistry with Medicinal Chemistry at Imperial College, where he continued his Ph.D. studies in biomimetic total synthesis of meroterpenoid natural products under the supervision of Prof. Anthony G. M. Barrett. He then moved to UCL as a research fellow in organic electrosynthetic chemistry, working with Dr. Jonathan D. Wilden to develop new electrosynthetic protocols. He is now back at Imperial College London, working with Dr. James Bull as a research associate in synthetic chemistry, focus on developing new synthetic protocols for transition metal catalyzed C-H activation of heterocyclic compounds. |

|
Zhenhao Zhong received his B.Sc degree in chemistry jointly from Nanjing Tech University and the University of Sheffield in 2019. Then he obtained his MRes degree in advanced molecular synthesis at Imperial College London. Zhenhao continued his Ph.D. studies at the same institution under the supervision of Dr. James Bull from October 2020, focusing on developing novel synthetic methods to synthesise sulfoximines and sulfonimidamides. |

|
Arianna Tota obtained the M.Sci. (summa cum laude) in Chemistry and Pharmaceutical Technology at the University of Bari (Italy) in 2015. In 2020, she obtained the Ph.D. in Chemical and Molecular Sciences under the supervision of Prof. Renzo Luisi. Her research activity is focused on the electrophilic nitrogen transfer to sulfur and the chemistry of nitrogen-bearing compounds. In 2019, she has been a visiting scholar at the Department of Synthetic Chemistry and Biological Chemistry, Kyoto University (Japan), working in the group of Prof. Aiichiro Nagaki. During this time, she was involved in the field of flow microreactor technology applied to organometallic chemistry. |

|
Leonardo Degennaro obtained the master degree in Chemistry and Pharmaceutical Technology in 1999 and the Ph.D. in Applied Chemical and Enzymatic Synthesis in 2003. In 2002 he was "visiting scholar" at the University of Groningen under the supervision of Prof. B. L. Feringa. In 2006 he was appointed assistant professor in Organic Chemistry at the Department of Pharmacy of University of Bari. In 2011 he has been "visiting assistant professor" at the University of Kyoto working in the group of Prof. J.-i. Yoshida. The research activity is aimed at developing new stereocontrolled synthesis by using small heterocycles and organometallic species, and microreactor technology |

|
Renzo Luisi is full professor of Organic Chemistry at the University of Bari (Italy). The research activity focuses on the chemistry of hetero-substituted organolithiums, the development of new synthetic methodologies, and the use of flow technology. He obtained the Ph.D. in 2000 under the guidance of Professor Saverio Florio. He has been visiting student at the Roger Adams Lab at Urbana Champaign in the group of Prof. Peter Beak and a visiting professor at the University of Manchester in the group of Jonathan Clayden. He is RSC fellow and recipient of the 2014 CINMPIS award Innovation in Organic Synthesis. |

|
Dr. James Bull is a University Research Fellow at Imperial College London. His research focuses on the development of synthetic and catalytic methods to access medicinally relevant structural motifs and heterocycles. He obtained his MSci degree from the University of Cambridge, then spent a year at GlaxoSmithKline. He returned to University of Cambridge for his Ph.D. with Professor Steven Ley. In 2007 he joined Université de Montréal as a postdoc with Professor André Charette. He started a Ramsay Memorial Fellowship at Imperial College in 2009, an EPSRC Career Acceleration Fellowship in 2011, and in 2016 was awarded a Royal Society URF. |

|
Ellie Plachinski obtained a B.S. in chemistry in 2020 from the Massachusetts Institute of Technology, where she conducted research in the laboratory of Prof. Alison Wendlandt. She is currently pursuing her Ph.D. in the Yoon Group in the Department of Chemistry at the University of Wisconsin-Madison. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved