Org. Synth. 2023, 100, 234-247
DOI: 10.15227/orgsyn.100.0234
Visible-Light-Promoted C-S Cross-Coupling Reaction for Synthesis of Aromatic Thioethers
Submitted by Yingjun Lan,
1 Junhua Xu,
1 Chern-Hooi Lim,*
3 Garret M. Miyake,*
2 and Bin Liu*
1Checked by Luca McDermott, Zachary Walters, and Neil K. Garg
1. Procedure (Note 1)
A. 1-(4-((2-Chlorophenyl)thio)phenyl)ethan-1-one (3). A single-necked (24/40 joint) 100 mL glass reaction tube with glass stopcock side arm is equipped with a Teflon-coated magnetic stir bar (3.0 × 1.5 cm, football-shaped) (Note 2) (Figure 1A). The reaction tube is charged with 4'-bromoacetophenone (3.98 g, 20 mmol, 1.0 equiv) (Note 3), cesium carbonate (9.77 g, 30 mmol, 1.5 equiv) (Note 4), and 2-chlorobenzenethiol (4.34 g, 30 mmol, 1.5 equiv) (Note 5), each in one portion. The tube is sealed with a 24/40 rubber septum and the tube is then placed under high vacuum (<1 mmHg) for 1 min and backfilled with nitrogen through the side glass stopcock three times. Dry dimethyl sulfoxide (50 mL) (Note 6) is added to the tube by a plastic syringe and needle through the rubber septum to give a yellow suspension (Note 7) (Figure 1B). The rubber septum is then sealed with electrical tape, the stopcock is closed, and the reaction tube is irradiated at 456 nm with one 40 W Kessil Lamp PR160 at a distance of 5 cm with stirring at 800 rpm. A fan is used to maintain the reaction tube at 23 ℃ (Figure 1 C).
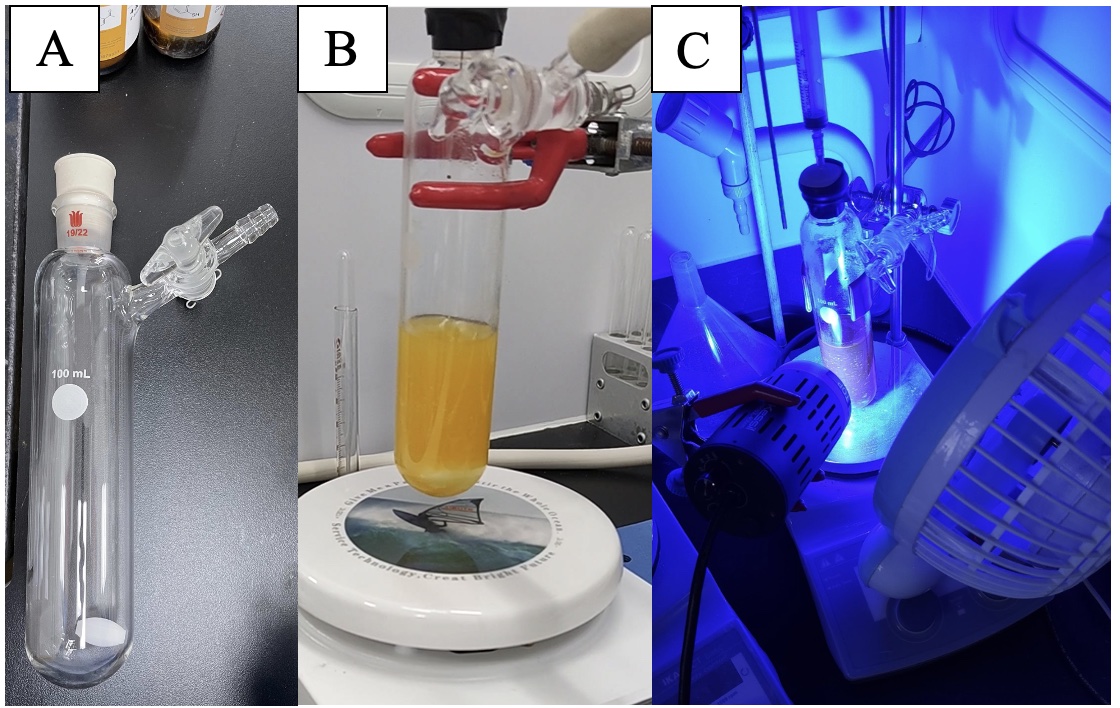
Figure 1. A) Reaction tube with stir bar; B) Reaction tube with stir bar and reagents; C) Reaction tube is irradiated at 456 nm with one 40 W Kessil Lamp PR160 lamps and a fan (photos provided by submitters)
After 5 h (Note 8), the tube is opened to the air, and the contents of the reaction vessel are transferred to a 500 mL separatory funnel. Water (10 mL) and 10 mL of EtOAc (10 mL) (Note 9) are used to rinse the reaction vessel to ensure a quantitative transfer. To the separatory funnel is then added 50 mL of water. The aqueous layer is then extracted EtOAc (3 × 75 mL). The pooled organic layers are washed with brine (20 mL), and then dried over 7 g of anhydrous magnesium sulfate (Note 10) for 10 min. After drying, the organic layer is filtered through a medium porosity fritted funnel, the magnesium sulfate rinsed with EtOAc (3 × 10 mL), and the organic layer concentrated on a rotary evaporator (30 ℃, 225 to 60 mmHg) to give the crude material, which solidifies as a tan solid.
Figure 2. A) Reaction mixture after 5 h at room temperature; B) Separated organic and aqueous layers during first extraction; C) Separated organic and aqueous layers during brine wash (photos provided by checkers)
The tan solid is dissolved in dichloromethane (3 mL) (Note 11) and submitted to flash column chromatography using a 5 cm diameter column with a length of 20 cm of silica gel (90 g) (Note 12). This column is compacted using silica gel with petroleum ether (200 mL) (Note 13), and the surface levelled. The crude material is transferred onto the compacted column with a pipette. The flask is rinsed twice with dichloromethane (1 mL) and the washings are added to the column. Once adsorbed, cotton is added to protect the silica.
Figure 3. Purification by column chromatography (photo provided by submitter)
The column is eluted with 1.5 L of a 5:1 petroleum ether:ethyl acetate solution. The tubes containing pure samples are collected (Note 14) in a 1 L round-bottomed flask and concentrated on a rotary evaporator (30 ℃, 225 to 30 mmHg) to afford 1-(4-((2-Chlorophenyl)thio)phenyl)ethan-1-one (3) as a white solid. The resulting solid is kept under high vacuum at room temperature for 4 h to afford pure product 3 (4.34 g, 83% yield) (Notes 15, 16 and 17) with 98% purity determined by qNMR analysis (Note 18).
2. Notes
1. Prior to performing each reaction, a thorough hazard analysis and risk assessment should be carried out with regard to each chemical substance and experimental operation on the scale planned and in the context of the laboratory where the procedures will be carried out. Guidelines for carrying out risk assessments and for analyzing the hazards associated with chemicals can be found in references such as Chapter 4 of "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
https://www.nap.edu/catalog/12654/prudent-practices-in-the-laboratory-handling-and-management-of-chemical. See also "Identifying and Evaluating Hazards in Research Laboratories" (American Chemical Society, 2015) which is available via the associated website "Hazard Assessment in Research Laboratories" at
https://www.acs.org/content/acs/en/about/governance/committees/chemicalsafety/hazard-assessment.html. In the case of this procedure, the risk assessment should include (but not necessarily be limited to) an evaluation of the potential hazards associated with
4'-bromoacetophenone,
cesium carbonate,
2-chlorobenzenethiol,
dimethyl sulfoxide,
ethyl acetate, anhydrous
magnesium sulfate,
dichloromethane, silica gel, petroleum ether,
1,3,5-trimethoxybenzene, and
CDCl3. In addition, hazards associated with use of a 40 W Kessil Lamp should be understood.
2. All reactions were conducted in oven-dried glassware under an atmosphere of nitrogen.
3.
4'-Bromoacetophenone (98%) was purchased from Adamas-beta and used as received. The checkers used
4'-bromoacetophenone (98%) obtained from Sigma Aldrich and used it as received.
4.
Cesium carbonate (98%) was purchased from Adamas-beta and used as received. The checkers used
cesium carbonate (99%) obtained from Sigma Aldrich and used it as received.
5.
2-Chlorobenzenethiol (98%) was purchased from Adamas-beta and used as received. The checkers used
2-Chlorothiophenol (99%) obtained from Sigma Aldrich and used it as received.
6.
Dimethyl sulfoxide (Water≤50 ppm (by K.F.), 99.7%, SafeDry, Safeseal) was purchased from Adamas-beta and used as received. The checkers used
dimethyl sulfoxide (anhydrous, ≥ 99.9%) obtained from Sigma Aldrich and used it as received.
7. In the event that the solid cake cannot be dissolved by the
DMSO, the entire reaction apparatus may be vortexed to disrupt the cake and generate a suspension.
8. TLC analysis of the mixture is shown below. The TLC plate was eluted with 5:1 petroleum ether:
ethyl acetate and visualized using 254 nm UV light. In order to remove
DMSO for TLC analysis of the reaction mixture, a small sample (<50 μL) is taken from the reaction mixture and added to a 1-dram vial with 1 mL of water and 1 mL of
EtOAc. The vial is capped and shaken, and then the TLC sample is taken from the organic layer.
Figure 4. Lane A contains a standard of 2-chlorobenzenethiol (2), Rf = 0.8. Lane B contains a standard of 4-bromoacetophenone (1), Rf = 0.45. Lane C is a co-spot of the two starting materials as well as the reaction mixture. Lane D contains the reaction mixture, showing primarily the desired product (3), Rf = 0.35 (photo provided by checkers)
9.
Ethyl acetate (AR, 99%) was purchased from Energy Chemical and used as received. The checkers used
ethyl acetate (>99.5%) obtained from Sigma Aldrich and used it as received.
10. Anhydrous
MgSO4 (98%) was purchased from Adamas-beta and used as received. The checkers used anhydrous
magnesium sulfate (>99%) obtained from VWR and used it as received.
11.
Dichloromethane (AR, ≥99%) was purchased from Energy Chemical and used as received. The checkers used
dichloromethane (>99.9%) obtained from Sigma Aldrich and used it as received.
12. Silica gel (Silica gel 60N, spherical and neutral, 0.08-0.048 mm) was purchased from Shanghai Haohong Scientific Co., Ltd. and used as received. The checkers used Silica gel (PC spherical silica gel, 50 μm, 60 Å) obtained from Silicycle and used it as received.
13. Petroleum ether (AR, ≥99.5%) was purchased from Energy Chemical and used as received. The checkers used petroleum ether obtained from Fisher Scientific and used it as received.
14. The material was collected in 13 × 100 mm fractions (10 mL per fraction). Fractions containing the product were identified by TLC analysis (using petroleum ether/
ethyl acetate (5/1, v/v) as eluent, where the product (
3) has an R
f 0.35. Fractions 49-108 contained the desired product, with UV active impurities eluting both immediately before (Fractions 37-47) as well as co-eluting (Fractions 61-87). Significant amounts of the desired product were observed in fractions 61-87, so these fractions were combined and their purity analyzed. These fractions were >98% pure, so they were combined with fractions 49-60 and fractions 88-108. Each test tube was washed once with
EtOAc (1 mL) to ensure quantitative transfer.
Figure 5. TLC analysis of column fractions (photo provided by checkers)
15. All NMR analysis was performed using
deuterochloroform (99.8%) as the solvent, which was purchased from J&K Scientific and used as received. The checkers used
chloroform-D (99.8%) obtained from Cambridge Isotope and used it as received.
16. Characterization data of
3:
1-(4-((2-Chlorophenyl)thio)phenyl)ethan-1-one, white solid; mp: 75-77 ℃; R
f = 0.35 (petroleum ether:
ethyl acetate, 5:1);
1H NMR
pdf (600 MHz,
CDCl3) δ 7.89 - 7.85 (m, 2H), 7.50 (dd,
J = 7.9, 1.3, 1H), 7.41 (dd,
J = 7.8, 1.6, 1H), 7.31 (td, 7.6, 1.7 1H), 7.28 - 7.23 (m, 3H), 2.57 (s, 3H).
13C NMR
pdf (150 MHz,
CDCl3) δ 197.2, 142.2, 137.2, 135.4, 134.7, 132.3, 130.6, 129.9, 129.2, 129.0, 127.8, 26.7
Figure 6. Isolated product 3 (photo provided by checkers)
17. A reaction performed on half-scale provided 2.28 (87%) of the product.
18. The purity of product
3 was determined using
1H qNMR
pdf analysis.
1H qNMR
pdf was performed using a mixture of
3 (17.7 mg) and
1,3,5-trimethoxybenzene (16.3 mg) (Sigma Aldrich, ≥99%, as an internal standard) in
CDCl3. The purity was calculated according to standard method as 98 wt%.
Working with Hazardous Chemicals
The procedures in
Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at
http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices.
In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red "Caution Notes" within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices.
The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein.
3. Discussion
Over the last 45 years, numerous procedures have been made for C-S bond formation.
2 In 1978, Migita and co-workers developed Pd(PPh
3)
4 catalyzed cross-coupling reactions between aryl iodides and thiophenols, which is one of the most powerful catalytic C-S bond-forming reactions.
3 In 2004, the Buchwald group used Pd(OAc)
2/1,10-bis(diisopropylphosphino) ferrocene (DiPPF) as a catalyst system for the cross-coupling of thiols and aryl halides, which promoted the substrate scope extensively.
4 In 2006, the Pd(OAc)
2/(R)-1-[(SP)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-
tert-butylphosphine (CyPF-
t-Bu) catalyst system was discovered by the Hartwig group for the coupling of aryl halides and triflates with thiols.
5 Nickel,
6 cobalt,
7 and iron
8 have also been used as a catalyst for the construction of C-S bonds.
In recent years, photocatalysis has emerged as a new approach for the preparation of C-S bonds. In 2013, Noël and co-workers reported the efficient direct conversion of anilines into aromatic thioethers with thiophenols by employing Ru(bpy)
3Cl
2·6H
2O as a photoredox catalyst.
9 Subsequently, nickel metallaphotocatalysis catalyzed cross-coupling of thiols with aryl halides was independently reported by the Johannes
10 and the Molander
11 groups. However, the direct C-S bond construction without transition metal catalyst remains underdeveloped.
In this context, we investigated and developed a visible-light-driven method for C-S bond formation without the use of transition metal catalysts.
12 This synthetic procedure tolerates a broad range of functional groups and a variety of substituted aryl halides can be transformed into the desired products in good yields (Table 1). Moreover, therapeutics such as indomethacin and fenofibrate can be converted to the corresponding thiolated compounds in good yields.
In summary, a practical method for the synthesis of aromatic thioethers is reported. This method features excellent functional group tolerance and good substrate scope.
Table 1. Substrate Scope. Reaction conditions: 1 (0.2 mmol), 2 (1.5 equiv), Cs2CO3 (1.5 equiv), DMSO (1.5 mL). Isolated yields are reported
Appendix
Chemical Abstracts Nomenclature (Registry Number)
2-Chlorothiophenol; (2) (6320-03-2)
4'-Bromoacetophenone; (1) (99-90-1)
Cesium carbonate; (534-17-8)
1-(4-((2-Chlorophenyl)thio)phenyl)ethan-1-one; (3) (869854-44-4)
Deuterotrichloromethane; (865-49-6)
1,3,5-Trimethoxybenzene; (621-23-8)

|
Yingjun Lan received his B.S. degree from Nanchang University in 2021. He is currently a second-year graduate student in the laboratory of Associate Professor Bin Liu at the Nanchang University. |

|
Junhua Xu received his B.S. degree from Jiangxi Science and Technology Normal University. He then moved to the Nanchang University where he is currently a second-year graduate student in Associate Professor Bin Liu's laboratory. |

|
Chern-Hooi Lim earned his Ph.D. in chemical engineering (2015) with Charles Musgrave at University of Colorado Boulder and conducted his postdoctoral research with Garret Miyake at Colorado State University. He is the founder and CEO of New Iridium Inc, which mission is to decarbonize the chemical industry via photocatalysis technology. Current focus includes steam cracking replacement and CO2 utilization. |

|
Garret Miyake earned his Ph.D. in chemistry with Eugene Chen at Colorado State University and performed postdoctoral research with Robert Grubbs at the California Institute of Technology. He is currently a Professor of Chemistry and the Dr. Robert Williams Professor in Organic Chemistry at Colorado State University. His laboratory develops organic photoredox catalysts and visible-light driven methodologies for the synthesis of small molecules and polymers. |

|
Bin Liu earned his Ph.D. in 2014 under the supervision of Prof. Bing-Feng Shi at Zhejiang University, where his research focused on nitrogen containing functional group-directed C-H functionalization. In 2016, he joined the laboratory of Prof. Garret. M. Miyake at University of Colorado, Boulder, as a postdoctoral researcher, focusing on visible light promoted reactions based on electron donor-acceptor complexes and synthesis of brush copolymers. In 2017, he moved to Colorado State University with the Miyake group. In 2021, he joined the School of Chemistry and Chemical Engineering at Nanchang University as a Professor. |

|
Luca McDermott was born and raised in San Francisco, CA. In 2020, he received his B.S. in Biochemistry from Tufts University where he carried out research under the direction of Professor Clay S. Bennett. In 2020 he began graduate studies at the University of California, Los Angeles, where he is currently a third-year graduate student in Professor Neil K. Garg's laboratory. His dissertations studies are primarily focused on total synthesis. |

|
Zach Walters was born and raised in Battle Ground, Washington. In 2022, he received his B.S. in Chemistry from Boise State University, where he carried out research under the direction of Professor Don Warner. In 2022, he began his graduate studies at the University of California, Los Angeles, where he is currently a first-year graduate student in Professor Neil K. Garg's laboratory. His studies primarily focus on total synthesis. |
Copyright © 1921-, Organic Syntheses, Inc. All Rights Reserved